
As we know, proteins are macromolecules usually composed of 20 amino acids linked together by peptide bonds. Upon entering the gastrointestinal tract (GI) of mammals, they undergo hydrolysis, breaking down into smaller fragments known as peptides. In the final stages of digestion, they form short peptides (consisting of two, three, or four amino acids in a chain) and free amino acids. Various industrial processes also break down proteins into peptides and amino acids, making them easier for the human body to process. There are multiple methods for protein hydrolysis, each resulting in a specific composition of peptides and amino acids (Hou Y. et al., 2017). However, for products intended for therapeutic use, only enzymatic (fermentative) hydrolysis is suitable. In general, most protein hydrolysates contain peptides with medium to long amino acid chains, which also applies to combinations with protein-based nutraceuticals using in vivo proteolytic enzymes (systemic enzyme therapy – SET). But the deeper the industrial protein hydrolysis, the higher the proportion of short peptides (di-, tri-, and tetrapeptides) in the hydrolysate. From a clinical pharmacology perspective, this means a reduced role for protein as a nutrient and an increased regulatory role for short peptides in metabolic processes as independent pharmacological agents. A typical example of determining the peptide-amino acid composition of a whey protein hydrolysate (WPH) during hydrolysis is shown in Fig. 1.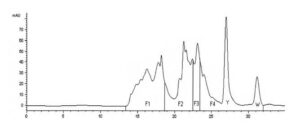
Figure 1. Chromatographic Profile of WPH (Whey Protein Hydrolysate) (citation: Silvestre M.P.C. et al., 2012): F1 — large peptides (> 7 amino acids); F2 — medium peptides (4–7 amino acids); F3 — di- and tripeptides; F4 — free amino acids; Y — tyrosine peak; W — tryptophan peak; substrate concentration 10%, enzyme — pancreatin, hydrolysis time — 5 hours, temperature ‒ 50°C, pH = 7.0; on the abscissa axis, time in minutes, on the ordinate axis, optical density (mAU — milliAdsorbentUnit).
The functional and nutritional properties of protein hydrolysates are determined by the qualitative and quantitative parameters of peptides, as well as the distribution of molecules with different molecular weights, which are traditionally determined chromatographically (Size Exclusion Chromatography). There are advanced methods for detecting low-molecular-weight peptides with a molecular mass of up to 1000 Da (Silvestre M.P.C. et al., 2012). The peptide and amino acid profile of the hydrolysate undoubtedly depends on the principles of enzymatic processing (choice of enzymes and conditions) and the initial substrate. As shown in Figure 1, WPH yields four fractions over time: F1 — at 13.5–18.5 minutes (large peptides with more than 7 amino acids); F2 — at 18.5–22.5 minutes (medium peptides containing 4 to 7 amino acids); F3 — at 22.5–23.5 minutes (di- and tripeptides); F4 — at 23.5–32 minutes — free amino acids. Literature data indicate that the initial stage of hydrolysis in the body leads to the formation of oligopeptides, containing 2 to 6 amino acids, and free amino acids (Frenhani P.B., Burini R.B., 1999). These peptides are then broken down into di- and tripeptides, which are absorbed along with free amino acids. From a clinical perspective, it is crucial to note that, according to these authors, the absorption of di- and tripeptides is more efficient than that of free amino acids, which, in turn, are absorbed better than peptides with a larger molecular weight. Based on this, an essential conclusion is drawn: the selection of a high-quality hydrolysate in practical terms should be based on a high percentage of di- and tripeptides (at least 15–16%) and free amino acids (about 47–48%), with a relatively small amount of large molecular weight peptides (about 25–26%).
The hydrolytic breakdown of plant proteins (vegaproteins) can provide a product with a high percentage of short peptides and corresponding metabolic activity in nutritional mixtures for all population groups, including vegans, vegetarians, and individuals with lactose intolerance. As clinical studies demonstrate, this can compensate for lower levels of BCAAs, especially leucine.
Short peptides not only get absorbed more easily by the intestinal walls but also, according to research (Zhanghi B.M., Matthews J.C., 2010), have specific transport mechanisms to cross the intestinal barrier into the bloodstream. This is a crucial factor in the metabolism of short peptides, which determines their fate. Within the small intestine, there exists a peptide transporter (PEPT1) responsible for the proton-dependent transport of extracellular di- and tripeptides through the apical membrane of enterocytes into these cells (Zhanghi B.M., Matthews J.C., 2010). However, due to the high activity of intracellular peptideases in the epithelium of the small intestine, it seems unlikely that a significant amount of short peptides in the gut lumen could enter directly into the portal vein or the lymphatic system. It is quite probable that a certain limited quantity of peptides is absorbed unchanged from the intestine into the blood through M cells, exosomes, and enterocytes, via trans-epithelial cell transport (Gardner M.L., 1982; Gardner M.L., Wood D., 1989). Peptides consumed with food can also activate signaling pathways, connecting the intestine with other internal organs, the endocrine, hormonal, and immune systems, influencing the entire body.
The sequence of actions of short peptides in the body after ingestion is roughly as follows:
It’s crucial to note that the formation and action of peptide factors are just as natural and metabolic processes as the functioning of amino acids. Attempting to explain the results solely from the perspective of amino acids interacting with cell receptors and being involved in intracellular metabolism cannot be considered satisfactory.