
The needs of pharmacological correction of the human activity in extreme conditions of hard work as well as sports of the highest achievements have become the real basis for the search and creation some tools of development and support of the organism’s adaptive potential, and means to optimize the use of organism’s reserve capabilities. The present review is devoted to the features of the properties and mechanisms of action of dipeptide L- alanyl-L-glutamine (ALA GLU), a relatively new means of peptide name, and hi the authors’ opinion according to the proved based spectrum of activity, can be attributed to the number of means which are able to increase the sustainability and resistance of the body to a wide range of extreme effects.
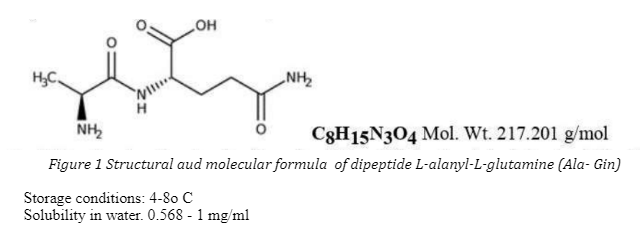
The substance L-alanyl-L-glutamine is a white crystalline molecule consisting of two amino acid residues: alpha-L-amino acids alanine and alpha-L-amino acids glutamine, covalently bound by a peptide bond that is similar to the current bond in proteins (Figure 1). Both amino acids are natural essential physiological substances, use as precursors of proteins thesis, and also are included in the 20 standard amino acids that play an important role in the synthesis of many natural proteins of the body. Both are important for global nitrogen metabolism in the body, gluconeogenesis, energy supply, plastic metabolism and runny other physiological processes.
Dipeptide L-alanyl-L-glutamine implements its action in different ways of introduction into the body and, depending on the way of entry, is carried out either the action of molecules AG, and/or dissociated molecules of alanine and glutamine. By oral or enteral introduction alanilglutamine acts locally in the gastrointestinal tract, helping to protect the integrity of the intestinal mucosa, and maintaining the barrier functions of the intestine. This reduces the possibility of bacterial translocation, the risk of infection, infection-induced inflammatory damage and infection-related symptoms such as diarrhea, dehydration, malabsorption and electrolyte imbalance. There is the fact of the ability of the actual dipeptide AG at different ways of introduction to support the integrative function of the intestine, accelerating the absorption of water and electrolytes from the intestine. a number of macro-and micronutrients, thereby providing a dehydrating effect and increasing the subsequent absorption of proteins and fats.
The chronological analysis of the ALA GLU action’s development by introduction internally allowed to say about a relative dividing dipeptide effects on (1) urgent- developing within an hour arid associated mainly with rehydration and improvement of the functions of excitable tissues, and (2) delayed- developing in hours and days after introduction into the body, and expressing by a stable anabolic arid anti-catabolic effects, increased immunity, increased glycogen reserves in muscles, etc. Besides this, it is noticed that ALA GLU is an effective supplier of alanine and glutamine[1.2.3] and thus mediates the differential action of these amino acids corresponding to the content needs of the body. Protein synthesis is carried out in the body constantly, arid interchangeable amino acid a-L-alanine (A- C3H2NO2) is included in many proteins, especially muscle, participating almost continuously in the turnover throughout life. The ability of alanine to easily convert into glucose causes the functioning of the glucose-alanine cycle-one of the main ways of gluconeogenesis in the liver.
Alanine is involved in the formation of muscle proteins, carnosine dipeptides, coenżyme A, the enzyme alanine aminotransferase (ALT), Pantothemc acid (vitamin B5). All this determines the basic physiological tasks of the present amino acid: maintaining a balance of nitrogen and a constant concentration of glucose in the blood. This amino acid is one of the most important sources of energy. In the human body, alanine is formed in muscle tissue from lactic acid. With intense physical activity, the lack of alanine stimulates catabolic processes in muscle tissues. alanine can be formed from other nitrogenous substances or during the decay of carnosine.
L-Glutamine (G- C5H10N2O3 amid monoamino dicarboxylic glutamic acid) is relatively an essential amino acid. The concentration of G in the blood is 500- 900 μmol/l, which is higher than the concentration of any other amino acid. G is a popular amino acid for dietary supplements, it is one of the most well-known candidates for the correction of the performance of persons subjected to increased physical activity, to optimize the physiological functions of athletes, as well as a known means of metabolic therapy in the clinic. Glutamine, being the most abundant free amino acid in the human body, along with glutamic acid, is a precursor to peptides, proteins, neurotransmitters, nitrogenous bases and is used as an energy source by various organs such as the intestine. This amino acid is involved in the realization of many functions, such as maintenance of cell proliferation (cell division), regulation of immunity and cellular activity, supporting of acid-base balance and regulation of gene expression [4.5,6].
In addition to its role as a component of protein G, it is important as a global nitrogen transporter, carries out the transamination of amino acids and participates in the formation of new amino acids. Glutamine deficiency reduces the proliferation of a number of cells and stimulates apoptosis (programmed cell death). The introduction of glutamine has a positive effect on glucose metabolism, including insulin resistance. It is proved that the deficiency of L-Glutamine leads to increased fatigue, reduced muscle strength, endurance and attention, increased reaction live arid a number of other adverse events that worsen performance in general and sports performance in particular. However, various studies in recent years have shown that the widespread use of L-Glutamine as a separate product is hampered by the peculiarities of its physical and chemical properties, such as: weak solubility and partial decomposition in an aqueous medium with the release of harmful ammonia gas, low thermal stability, pronounced dependence on the pH of the solvent, instability in an acidic medium.
The literature presents a large factual material on the positive effects of L- Glutamine in the dosage range of 0.2-0.4 g/kg/day in athletes and persons engaged in regular exercise [7.8, 9.10. etc.].
At the same time, a number of publications reported that there is no evidence of the effectiveness of L-Glutamine and hypertension in the training process. especially in persons with highly adaptive nutritional status[ 10, 11].
It can be thought that these differences are primarily due to the large variability of the studied groups of individuals, differences in methodological approaches, the incompatibility of the recorded parameters, as well as the instability of L- Glutamine in the acidic environment of the stomach and other factors. However, glutamine is used in clinical practice as an oral, parenteral or enteral Supplement in the form of a separate amino acid or in the form of glutamine-containing dipeptides. which, as it turned out, have a number of advantages coutpaced to G.
In particular, AG and other dipeptides exhibit greater physical and clinical resistance, provide better delivery and in greater quantities to the organs, tissues and intercellular space of glutamine and alanine. Thus, the voter solubility of AG at room temperance arid stability in a solution substantially higher than the initial free amino acids alanine (A) and glutamine (G), and higher than that widely used in sports arid clinical pharmacology dipeptide Glycylglutamine (GG). The ranked number of substances under consideration, in order of decreasing their solubility in water, is as follows:
AG (alanyl- glutamine, AG) >Alamn >GG (glycyl-glutamine, GG) > Glutamine
Characteristics of physical and chemical properties of these substances are extremely important for the assessment of their pharmacological properties arid should be taken into account for the prediction of digestibility in a particular cry of introduction into the body, evaluation of biological distribution in the body, calculation of dosages of ingredients and for the correct comparison of physiological efficiency. Thus, the water solubility of L-Alanyl-L-Glutamine (AG) is 15 times higher,
al-Glycyl-L-Glutamine (GG) is about 4 times higher than L- Glutamine. Along with this, instability of L-Glutamine (G, GLU) in aqueous solutions, low resistance in the acidic environment of the stomach and relatively slow and incomplete absorption in the intestine were revealed (Table I).
Table 1: Comparative characteristics of physical and chemical properties of L- Glutamine and its dipeptides *
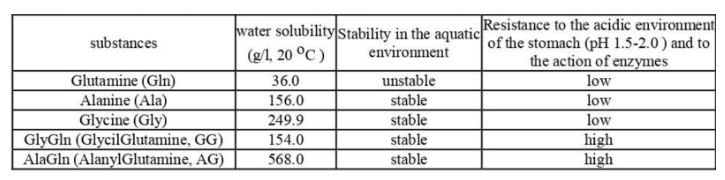
* – P .Furst, 2001 [ 12 ; Yasufumilmamoto, 2013[13] with additions
It has been proved that within the first hour at normal temperature of the body is destroyed about 50% of ingested L-Glutamine, whereas AG is stable for at least four hours, creating an environment conducive to the full absorption in the intestine. In addition, the therinostability of AG is much higher than that of glutamine, which decomposes upon heating with the formation of toxic ammonia.
These and other features of hypertension, which will be discussed below, are the advantages of dipeptide as a pharmacological agent. So, due to the stability of AG in aqueous solutions, along with dry and powdery forms as dietary Supplements (usually dry crystalline powders or more often encapsulated powders, un- der the name “L-Glutamine”, “L-Glutamine”, “Sustamin”), drugs have been created in the form of a concentrated 20% aqueous solution of AG, for parietal nutrition (drug “Dipeptive”, and its analogues), which is intended for drip intravenous (in) infusion into the Central veins after compatible infusion solution. The dose depends on the severity of the patient’s hypercatabolic state and amino acid requirements. The recommended maximum daily dose for the drug “Dipeptiven” is 2.5 ml/kg, which is equivalent to 0.5 g/kg of pure L- alanyl-L-glutamine, i.e. 3,5 g/day for a person weighing 70 kg. After the on/in the introduction of L-alanine- L-glutamine is rapidly hydrolyzed in plasma to form alanine and glutamine. The half-life (T1/2) of L-alanine- L-glutamine is 2.4-3.8 minutes. According to the instructions, the introduction of dipeptide AG regulates nitrogen balance and protein metabolism, maintains the intracellular pool of glutamine, correct catabolic response, improves immune function, reduces the frequency of infectious complications, restores the function of the intestine [14]. Infusion of dipeptide L-alanine-L-glutamine leads to a rapid increase in the concentration of glutamine and alanine in organs and tissues, primarily in muscles and liver, during the entire period of infusion and only trace amounts of dipeptide can be detected in plasma. Recommended dosage for oral administration of L-alanyl-L-glutamine (sustamin) 1-3 grams per day (Glutamine — up to 15 g/day). Additional studies are needed to determine the optimal dose. The method of application of L-alanyl-L-glutamine can be similar to the method of application of glutamine [15]. A comparative study of pharmacokinetics of oral introduction of AG and G demonstrated that in L-Glutamine equivalent doses – dipeptide (89 mg/kg) to a greater extent than the free form of L-Glutamine (60 mg/kg), provides a long and significant increase in the concentration of L-Glutamine in blood plasma. The initial concentration of L-Glutamine is 475 ±108 μmol/l. After 30 minutes of L-Glutamine administration the amino acid concentration increases by a maximum of 179 ± 61 pmol/l with a return to the initial values after 2 hours. The mean area under the concentration curve (AUC) between 0 and 4 hours was 127 ± 61 μmol·h·1-1. After the introduction of AG, the peak of increasing the concentration of L-Glutamine in plasma was +284 ± 84 μmol/l (to the base values), which is 59% higher than after the introduction of L-Glutamine (P < 0.05). The duration of the increase in the concentration of L- Glutamine was also longer in the case of dipeptide, and the average value of AUC was 284 ± 154 μmol·h·1-1, which is higher in two times more than in the case of L- Glutamine (P < 0.05). In a clinical study, it was found that the ingestion of AG according to the scheme – once 20 g, 5 times a day for 4 g, not only exceeds the hee form of L-Glutamine in the rate of absorption in the in- testine by more than 2 times, but also retains this ability in chronic inflammation and reduced gastric secretion. Such features of AH properties indicate the prospects of practical application of dipeptide in clinical and sports medicine [14,16].
In comparative terms, hypertension is more stable in the gastrointestinal tract, better absorbed in the digestive tract and absorbed by the liver orally than flee L- glutamine, often used intravenously in clinical nutrition [17].
Moreover, with oral introduction, AG is more effective for increasing glutamine levels in muscles than the equivalent amount of glutamine. Perhaps this is due to the fact that after intravenous hypertension quickly hydrolyzed into molecules A and G, and after oral introduction the molecules of AG work in place in the small intestine of the digestive tract, where the absorption occurs, then a small part of the molecules of dipeptide and free amino acids is transported with lymph in a large circle of blood circulation, and the main part of portal blood is transported to the liver. Further, amino acids, it is possibly that short peptides and proteins synthesized in the liver are transported to other tissues.
Intensive physical activity is a powerful physiological stress, which during the period of the stress factor limits and even turns off the ability of the intestine to fully absorb proteins, fats and carbohydrates, reduces their maximum transportable volume. Chronic intensive physical activity often leads to a number of gastrointestinal disorders, especially in those sports that require increased resistance [18].
Therefore, AG is recommended to be used both before prolonged exercise to improve the absorption of electıolytes and increase enduıance, and after intense loads to restore the absorption capacity of the intestine. The method of use of L- alanyl-L-glutamine can be similar to the method of use of glutamine (in matters of dosage, regimen and indications).
It has to be noted that both alanine and glutamine are important essential substances glutamine is the most common amino acid in free form in human cells and intercellular space. G is synthesized in the human body in a sufficient amount for a normal lifestyle, however, not being an essential amino acid, because of its Central role in global mtıogen metabolism and participation in many regulatory and metabolic processes is consideıed conditionally indispensable [19 , 20 ]. In addition to its role as the most common protein component and importance in amino acid transamination, glutamine is an important component of various metabolic processes. Glutamine is metabolized in almost all organs and tissues and can be classified as a time regulator of amino acid balance. In extracellular fluid, glutamine is about 25%, and in skeletal muscle is more than 60% of the total pool of free amino acids. The transmembrane concentration gradient in muscles is about 34:1 (intracellular/ extracellular fluid). The concentration of free glutamine varies greatly in different organs and tissues. It is important that the blood plasma contains only a very small part of the free glutamine in the body and the concentration of this amino acid in the plasına does not directly depend on the intracellular concentration, so the concentration of glutamine in the plasma can not serve as a marker of glutamine in the body [26]. The total content of glutamine in the body is mainly determined by the proportion of this amino acid in the protein: 4.3±0.6 g per 100 g of muscle protein. Muscles are the main endogenous source of glutamine. Taking into account the fact that muscles make up 40% of body weight, it is believed that the total content of glutamine in muscles in a man of weight 70 kg is approximately 240-280 g [2, 12].
At extreme physical pressure or critical pathological conditions, free glutamine is depleted very quickly, the body compensates for the level of free glutamine primarily due to the breakdown of muscle proteins and increased glutamine synthesis. The reason for the development of glutamine deficiency — a large mımber of metabolic reactions and functions that directly or indirectly depend on glutamine, as well as high and rapidly increasing demand for it in rapidly growing cells.
Moreover, glutamine serves as an interorgan nitrogen Transporter in the body. Approximately 1/3 of all nitrogen is transported in the blood in the form of glutamine [21]. Thus, the endogenous reserves of glutamine and the possibility of its synthesis are suclı that in conditions of exhausting loads or aggıessive stress relatively quickly develops relative deficiency G.
Most of the nitrogen consumed by muscles is used in muscle cells to synthesize glutamine, which is a nontoxic carrier of ammonium from peripheral tissues to internal organs. Glutamine is the main substrate for the synthesis of urea in liver hydration genesis in the kidneys lu mitochondria with glutaminase glutamine can turn into glutamate with the formation of ammonium. In general, Glutamine, as an interorgan nitrogen carrier, is important in the excretion of protein metabolism products (urea, creatinine, uric acid, purine bases, etc.) and maintenance of acidbase homeostasis in the kidneys with the renal isoenzyme glutaminase glutamine is used for ammoniogenesis with the consumption of H*ions.
Additionally, it has to be noted that both amino acids G and A are vital not only for the exchange of nitrogen storage of glucose. Moreover, the functions of alanine and glutamine are realized not only in parallel, but they are often interrelated and synergistic: alanine is the most important amino acid involved in the transfer of nitrogen from the muscles to the liver. Once there, the carbon skeleton of the amino acid can be converted to glucose as part of the glucose-alanine cycle [22].
Both amino acids also improve hydration in synergistic ways. Apparently, Alanine affects hydration and cell voluıne by increasing intıacellular potassiınn concentrations. thereby drawing water into the cell. Glutamine also affects the volume of cells, helps to maintain the overall acid-alkaline balance of the body, due to aminoma, which is cleaved from the amino acid and delivered to the kidneys, affects the transport of water. In response to heavy physical exertion or other forms of physiological stress, both amino acids are released from the muscles in significant concentıations. If the concentrations of these amino acids in the muscles are not restored, then resistance to physical activity, irrational recovery, decrease in the functions of the immune system, muscle growth. General hypofunction may decrease. Healthy people of weight about 70 kg have from 70 to 80 g of relatively free glutamine distributed throughout the body.
In quantitative terms, skeletal muscles are the most significant pool of glutamine [23]. Using isotopic and pharmacokinetic methods, it was found that the endogenous production of glutamine ranges from 40 to 80 g / day [24-26]. In plasma obtained from blood samples, the concentıation of glutamine varies from approximately 500 to 800 μm/ 1 (values recorded after 12 hours on an empty stomach), which is about 20% of the total pool of free amino acids in the blood [27]. In tissues such as the liver and skeletal muscles, the concentration of glutamine is even higher than in plasma, which is about 40-60% of the total amino acid pool. As in plasma, and in tissues especially muscular, glutamine concentration in 10-100 times higher than any other. The total reserve of glutamine muscle reserve is relatively small (in a person with a mass of 70 kg about 240 g), the stress-induced decay of I kg of muscle tissue provides only 9 g of glutamine, so the catabolic release of glutamine is limited and insufficient for prolonged physical pressure of depleting action [23].
Thus, skeletal muscles play a critical role in glutamine metabolism, as muscles are one of the most common tissues in the human body [28]. intramuscular glutamine content corresponds to 50-60% of the total free amino acids in the tissues of skeletal muscles. Approximately 80% of glutamine in the body is found in skeletal muscles, and this concentıation is 30 times higher than in human plasma [29,30]. The concentration of free amino acids in muscle tissue depends on the type of muscle fiber. Studies on rat skeletal muscle showed that glutamine concentrations were three times higher in slow muscle fibers (type 1 fibers) than in fast muscle fibers (type 2 fibers). High concentration of glutamine in the slow twitch muscle fibers due to the high activity of the enzyme GS and the availability of ATP for the synthesis of glutamine [31].
Glutamine is an important source of carbon and nitrogen for various substrates in the body. G is used directly for protein synthesis and serves as a precursor for the synthesis of other amino acids. G — donator of nitrogen for the synthesis of monosaccharides, purines and pyrimidines used for the synthesis of nitrogenous bases that are part of deoxyribonucleic (DNA) and ribonucleic (RNA) acids necessary for cell proliferation and protein synthesis [32).
When critical voltage occurs functional redistribution of the supply of organs plasticheskie and energy resources, develop state hypercatabolism and hypermetabolism imbalance between the production and consumption of glutamine.
The small intestine is the main organ that consumes glutamine. Under stress, the use of glutamine by the gut increases, which increases its deficiency. Today it is proved that glutamine is an absolutely necessary substantiate to maintain the structure and function of the intestine (33, 34), especially in conditions when there is damage to the intestinal mucosa, deterioration of barrier function and, consequently, an increase in the degree of translocation of bacteria and toxins into the bloodstream [33-35). If hyper catabolism is not corrected, the risk of multiorgan failure increases. It is suggested [34] that increased consumption of glutamine under stress can save glucose for organs that obligate use it for energy: the brain, red blood cells, bone marrow and granulation tissue. It is shown that rapidly dividing cells, including cells of the intestinal mucosa, pancreas, pulmonary alveoli and cells of the immune system, use glutamine for energy and plastic needs. Glutamine is the main source of energy for cells of the gastrointestinal tract (enterocytes, colonocytes) (33).
The physiological state of the small intestine is highly important for the absorption of substances primarily of protein nature, because it is in this part of the gastrointestinal tract that acid degradation of polypeptide molecules is completed, their enzymatic hydration and absorption occurs: oligonucleotides, short peptides (tripeptides, AG and other dipeptides), as well as amino acids (Figure 1). An important role in the breakdown and absorption of peptides play borderline enterocytes, located on the villi of the intestinal epithelium. These cells are involved in almost all stages of digestion, including parietal and membrane.
LUMEN OF THE INTESTINE
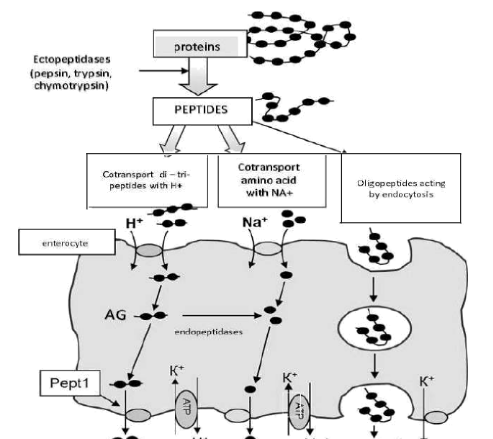
Figure 1 Scheme of transport of amino acids and substances of peptide nature (oligopeptides, ditripeptides) through the cells of the small intestine (enteroy tes) in the portal erm of the liver and lymphatic bed. Transport AG is a proton (H+)- bound cotransporter PepT1.
It is now established that polypeptides are broken donor under acid and enzymatic action in the lumen of the intestine to oligopeptides, peptides consisting of two or three amino acids to the amino acids. Forming di- and tripeptides accumulate in significant amounts in the cytosol of cells (enterocytes) of the intestinal mucosa. Some of these peptides are hydrolyzed to free amino acids, but some di-and tripeptides are able to persist in cytosol hydrolysis and be transported intact through the basolateral membrane, joining the transport protein Peptl — proton (H+)-bound cotransporter (36). Getting mainly into the venous blood of the portal bloodstream and further into the liver and lymph. Thus, with oral or enteral introduction. AG acts locally in the gastrointestinal tract both to protect the integrity of the intestinal mucosa and to maintain the barrier functions of the intestine. These effectives are the basis of the positive influence of AG — maintaining an/or restoring integrative function of the intestine, the normalization of the absorption of nutrients, prevention and inhibition of the development of wasting syndrome (increase of lean body mass — LBM), a correction of the balance of the metabolism by increasing the anabolic and inhibition of catabolic processes in muscle, lung arid nerve tissues.
In this regard, among the “urgent effects” of dipeptide AG, the actions associated with the acceleration of the processes of rehydration of the body at high prolonged physical pressiue are justified that there is a presentation of efficiency for a longer time and with high efficiency. Also AG provides a significant ergogenic advantage by increasing the time of endurance under moderate hypohydration stress (37- 40). At the satire time, in a number of works it is noted that AG not only exceeds the free form of L-glutamine in terms of the rate of absorption in the intestine more than twice, but also returns this ability in systemic chronic inflammation and low secretion of the stomach. In general, such features of the action of hypertension can be of direct practical importance for the use of dipeptide in clinical and sports medicine.
A number of studies have found that rapidly dividing cells, including cells of the intestinal mucosa, pancreas, pulmonary alveoli and cells of the immune system, use glutamine for energy and plastic needs. And for cells of the gastrointestinal tract (enterocytes, colonocytes) glutamine is the main source of energy [33, 41].
During the intracellular oxidation of glutamine is formed ATP which is the most significant molecule form of conservation arid use of chemical energy in cells, the total amount of stored energy depends on the availability of glutamine and the degree of its oxidation. In stress environment, this is mainly determined by the level of glutamine deficiency, the availability of glucose as an alternative source of energy in some tissues and the phase of the cell life cycle. For example, lymphocytes use glutamine for energy to a greater extent after mitogenic stimulation (41). Under physiological conditions, glutamine oxidation gives about 1/3 of the energy in rapidly dividing cells [42], with strong stress and pathological reactions, glutamine oxidation can increase.
The functioning of the immune system is also envied by the availability of glutamine. Stress, causing glutamine deficiency, disrupts the immune system. The consumption of glutamine by cells of the immune system is increased by 10 times compared to other cells [43, 44, 45].
Glutamine is an indispensable substrate for the nominal functioning of humoral and cellular immunity. Thus, in vitro studies have shown that the lack of glutamine in the tissue culture environment severely limits the ability of lymphocytes to respond to mitogenic stimulation [46]. In addition, some inflammatory mediators (interleukins, etc.) and glucocorticoids increase the activity of lymphocyte glutaminase, including in mesenteric lymph nodes, which leads to increased utilization of G.
Consequently, the use of a unique intestinal Transporter exogenously in the form of a molecule of the dipeptide AG can cover the possible relative scarcity arid more efficiently to increase the resets e amount of glutamine in the bloodstream extracellular space in skeletal muscle and cells in comparison with conventional free G. With the introduction of AG is supported by a higher level of conc entiation in the glutamine compartments: blood plasma, lymph, intercellular space arid skeletal muscle, compared with the introduction of a free City. This feature of the biodistribution of AG is very important because up to 65% enter oral by free G can be destroyed before it reaches the muscles. It can be assumed that in a healthy body there is a multiparameter dynamic homeostasis of glutamine, it is balanced and configured in such a way that an unstable reserve amount of G is maintained. Current needs are met by the reserve and release of glutamine hour the muscles and lungs through the breakdown of its own proteins, arid replenishment is due to external revenues and increased synthesis of glutamine denovo. G is actively spent primarily on maintaining a normal structure and the functions of the intestinal mucosa, hepatic ammoniogenesis, proliferation of lymphocytes, other cells of the immune system, muscle function. A decrease in intramuscular glutamine concentration causes a significant increase in muscle protein breakdown. The total muscle supply of glutamine is relatively small (about 240 g), the stress-induced breakdown of 1 kg of muscle tissue provides only 9 g of glutamine, so the catabolic release of glutamine is limited end insufficient with increasing needs. During catabolic stress, a patient with weight 70 kg has a higher intake of glutamine by the intestinal mucosa, kidneys, and immune system than the body can compensate by breaking down its own muscles and increasing glutamine synthesis by about 12 g/day. The real need for glutamine under stress and extreme physical pressure is not less than 18-22 g/day [45].
During the search, in particular it was found that polyunsaturated fatty acids (PUFA), such as omega -3, omega -6, omega-9 and others (more than 11) affect the metabolism in the body, including at the cellular level. It turned out that PUFA is not synthesized in the tissues of the body, but necessary for its life. In particular. PUFA protects cells from premature aging and helps to preserve their genetic information. Regulate fat metabolism and vital activity of beneficial bacteria living in the intestine. In 2004, the FDA (US Food and Drug administration) recognized that consumption of some omega-3-PUFA (eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA)) may reduce the risk of cor-onary heart disease. In the body of peroxide oxidation of polyunsaturated fatty acids counteract the antioxidant system including the glutathione Tripeptide, which is synthesized from glutamine [47-52].
It has been approved that dietary supplements, including fish oil, containing polyunsaturated fatty acids omega-3PNZHK, eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), reduce mortality inpatients with chronic heart failure[52]. The effect of PUFA was manifested by improvement of cardiac function indices, exercise tolerance and circulating cytokine levels (TNFa, IL-1 and IL-6) in patients with chronic stable heart failure[53].
Moreover, as noted above, G is not only involved in maintaining the homeostasis of nitrogen balance due to changes in the intensity of proteolysis of the muscle, but also in maintaining synthesis of the powerful anti-oxidant and regulator of cellular integrity of membraneprotective, probably warning the rapid oxidation of PUFA[55-58].
In blind, randomized clinical studies in patients with progressive heart failure ( HF), which was caused by the progressive development of metabolic disorders, inflammation and atrophy in the myocardium and skeletal muscles, it was noted that there is a clear metabolic transition from the use of normal fatty acids to the use of glucose as the main energy substrate for the generation of ATP in the tricarboxylic acid cycle (CTC). It is assumed that this switching is the result of changes in the genetic program with a decrease in the expression of the enzymes carnitine palmitoyltransferase (CPT) -1, acyl-COA-dehydrogenase with an average chain length (MCAD), citrate synthase, and consequently the use of PUFA[55,58].
This metabolic switching is observed in skeletal muscles with increased (glycolytic) type II muscle fibers and reduced (oxidative) type I fibers [60]. It is assumed that this internal metabolic transition in skeletal muscles contributes to the development of physical exercise’s intolerance.
In the study [55], patients received for longterm (1 and 3 months) ingestion of either a combination of 8 g / day AH and fish oil (source of PUFA) 6.5 g 1 day, or a combination of safflower oil and milk powder (placebo) in caloric equivalent amounts. Studies have set that the combined Supplement of 1-alanyl-l-glutamine and PUFA did not improve test exercise, muscle properties (biopsy analysis) or muscle function, but increased muscle mass and improved quality of life in patients with chronic stable heart failure.
These and other data indicate the potential usefulness of the effects of high_ doses of PUFA and the effectiveness of simultaneous action of dipeptide AG supplementation in patients with chronic stable heart failure [61-62]. The question of the mechanisms of interaction and positive influence of AG and polyunsaturated fatty acids on the growth of muscle mass remains: it is not clear whether AG (actually a dipeptide) Transporter of fatty acids into the cell, whether indirectly by influencing the aggregation of chains of fatty acid molecules with the formation of micelles and those facilitating their absorption in the intestine, or affects the absorption and biosynthesis of other physiologically active substances inside the cells.
Cellular mechanisms of action of PUFA are intensively investigated, but have not yet been determined, but it is believed that omega-3 eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) can act both directly and through metabolites such as eico-sanoids[61,62].
In this regard, there is a reason to consider the mechanisms of modulation of fatty acid oxidation in the intercellular space, within the cell membranes and in the cell cytosol by glutathione as a probable sphere of the control action of AH on the processes of catabolism and absorption of PUFA. The Tripeptide glutathione is a powerful systemic antioxidant, is able to predictably influence the utilization of fatty acids. Although there is currently no scientific evidence of the direct impact of PUFA consumption on the performance and level of physical fitness of athletes, however, some of the identified properties of omega-3 and omega-6 PUFA, indicate the possibility of direct or indirect positive effects of fatty acids on the function of a number of organs and systems during training, accelerate recovery, reduce the short-term or long-term use of the consequences of muscle and ligament injuries, reduce inflammation, stabilize the cardiovascular and respiratory systems at rest and during exercise. AG favors such action of PUFA [63-66].
In its turn, glutamine plays an important role in the regulation of glutathione synthesis. It is indicated that glutamine, increasing the formation of glutathione, causes cellular anabolic effects by increasing the volume of cells. On the stress, when the content of free radicals damaging cells is increased in some tissues, the need for glutamine increases [67].
The possibility of direct influence of food intake of enriched AG on increase of glutathione level (GSH) in blood serum and liver tissue was shown experimentally on animals. The relationship between arterial hypertension and the biosynthesis of glutathione (GSH) has been studied as well as the role of communication in protecting the liver. Twenty male Wistar line rats were used, they were randomly divided into two groups: one received standard parenteral nutrition (STD), and the other – with or without hypertension for 7 days. Blood and liver tissue samples were examined after peritoneal injection of hepatotoxin 5-fluorouracil (5 -FU) .
It was found that the concentration of glutathione (GSH b) in serum was significantly higher in the AG group than in the group with STD and the content of GSH in liver tissue was 36.2% higher than in the group with a standard diet, Rats in the AG group had a lower increase in liver enzymes after administration of 5 -FU, indicating hepatoprotective effect of dipeptide. I. e. standard food supplemented with AG can protect liver function by increasing the biosynthesis of glutathione and preserving glutathione reserves in liver tissue [68].
Reduced glutathione (GSH) is a Tripeptide composed of the amino acids L- glutamate, L-cysteine, and glycine, it is the most effective antioxidant supporting the intracellular redox potential (or redox potential), which is the ratio of reduced o oxidized (GSSG) forms of glutathione (GSH/GSSG). Maintaining the optimal GSH>>GSSG ratio In a cell is critical for its normal functioning and survival. Glutathione system is a trap of flee radicals, in particular, prevents the development of intracellular oxidative stress. GSh protects cells from damage caused by the production of free radicals during physical activity, especially when their levels exceed the body’s ability to defend against them, The lack of GSH puts the cell at risk of oxidative damage[69-71].
Glutathione system of redox potential not only provides resistance of cells to oxidative stress, but also participates in mechanisms of utilization of polyunsaturated fatty acids with subsequent synthesis of cytokines and eicosanoids, Eicosanoids that oxidized derivatives of polyunsaturated fatty acids – eicosatrienoic (C20:3), arachidonic (eicosatetraenoic, C20:4), timnodonic (eicosapentaenoic, C20:5), Eicosanoids are synthesized from omega-3 (co-3) and omega-6 (co-6) fatty acids. So, omega-6 are Pro-inflammatory eicosanoid. Edible sources of polyunsaturated fatty acids are vegetable oils, fish oil and omega-3 fatty acids. Eicosanoids are involved in a variety of processes, such as muscle growth, irritation and immune responses to toxins and pathogens. Eicosanoids are formed in almost all cells of the body. But GSH glutathione depletes relatively quickly, especially in conditions of glutamine deficiency, and recovers relatively slowly [72-75].
Especially intensive glutathione is consumed in the kidneys, liver, intestinal mucosa and macrophages. The half-renewal period of GSH (T1/2) in blood plasma is 2 min; in the kidneys 30-50 min, in the liver, intestinal mucosa and macrophages 2-3 h; in the brain; erythrocytes, spleen, lungs 2-4 days; in the lens of the eye 2- 8 days [76].
It can be expected that AG not only can affect the absorption and utilization of polyunsaturated fatty acids, synthesis of cytokines and eicosanoids, the synthesis of glutathione, but also as a supplier of glutamine on the synthesis of fatty acids and membrane phospholipids occurring with the participation of metabolites of glutamine, including the substrate of the Krebs cycle acetyl-coenzyme A, which provides acetyl group. Glutamine intake into muscle and liver cells is believed to increase their hydration and serves as an anabolic proliferative signal [77]. Oral administration of AG can change the whole metabolic response of the body to stress.
AG can also be used to correct energy metabolism: both to improve gluconeogenesis in the liver and to Deposit glucose in the form of glycogen. In addition, the intracellular oxidation of glutamine produces ATP, which can be considered as an alternative source of energy at high physical pressure.
Analytical review of scientific publications on the properties, mechanisms and effectiveness of the action of dipeptide L-alanyl-L-glutamine (AG) allows us to conclude that AG is a promising product that will be in demand in sports and clinical medicine, as well as in a wide range of individuals as a practically safe soft adapto protective and adapto genic means to increase human resistance to extreme physical pressure. In a situation with low and moderate physical pressure, its effectiveness will be unobtrusive against the background of a good quality of life, the maintenance of which the reception of hypertension will contribute. Since glutamine, glutamic acid and alanine are at the center of almost all types of metabolism, matching protein, carbohydrate and fat metabolism, as well as the exchange of nucleic acids and biogenic amines, the lack of these key “characters” of the body is formed relatively quickly, if the intensity of physical pressure leads to depletion of reserves. Potentially, due to its exceptional physicochemical and biochemical properties, AG can become one of the best means of preventing and/or eliminating deficit States, as well as a good means of targeted metabolic correction. At the same time, the scientific substantiation of the spectrum of pharmacological activity, the substantiation of the mechanisms of action and the development of recommendations based on the principles of evidence-based medicine are yet to come.
 IPH – PEPTIDES
Read more
IPH – PEPTIDES
Read more