
The pancreas is comprised of two distinct components: the exocrine pancreas, a repository of digestive enzymes, and the endocrine islets, which are the source of the essential metabolic hormone insulin. Human islets have a limited capacity for regeneration. The loss of islet β-cells in conditions such as type 1 diabetes, and the disruption of enzymatic balance seen in type 2 diabetes, necessitate therapeutic intervention. A leading strategy for restoring β-cell mass is the generation and transplantation of new β-cells derived from human pluripotent stem cells. Additional approaches include stimulating the endogenous proliferation of β-cells, reprogramming non-β cells into β-like cells, and harvesting islets from genetically modified animals. Together, these approaches form a comprehensive toolkit for pancreatic regeneration. In cases of enzymatic imbalance, hypoglycemic drugs are administered.
The pancreas plays a critical role in the proper metabolism of nutrients, performing both endocrine and exocrine functions. Over the last two decades, our understanding of pancreatic development during embryogenesis has expanded significantly, largely thanks to research on model organisms. The molecular basis for pancreatic origin decisions and cell differentiation is well understood. However, the mechanisms controlling the organ’s three-dimensional morphogenesis, crucial for therapeutic strategies, are less well-studied.
The pancreas is central to digestion and absorption, as well as the utilization and storage of energy substrates. As mentioned earlier, it consists of two structurally distinct but functionally integrated glandular systems: the exocrine and endocrine pancreas, both of which arise from the outgrowths of the primitive gut. The secretion of the exocrine pancreas is modulated by neural and hormonal signals, especially in the form of numerous gastrointestinal peptide hormones.
Given the above, investigating the properties of drugs capable of preserving pancreatic function is considered timely. This article will present a brief overview of research findings on the effects of the peptide IPH LGAT, which may contribute to optimizing pancreatic function and, consequently, offer additional preventive benefits for overall health. The increase in levels of local and systemic biomarkers, which are released upon peptide application and possess protective properties, underscores the importance of their use in maintaining and restoring organ functions at any life stage and in any disease context.
Objective: To explore the potential of the peptide complex IPH LGAT.
In the first part of the study, we conducted tests on cell materials. We chose embryonic stem cells, which are pluripotent and capable of differentiating into all three primary germ layers: ectoderm, endoderm, and mesoderm, from which organs and the pancreas subsequently develop, to assess proper ontogenesis and cytoprotective properties. The expression of BMP and FGF genes, as well as the PDX-1 gene, was studied using immunofluorescence (1:150, Abcam). The study groups included measurements of molecule expression before the study began, a control group (adding nutrient medium, incubating with serum albumin), adding the control dipeptide Glu-Trp at a concentration of 100 micrograms (µg), and adding the IPH LGAT peptide at a concentration of 100 µg. For measuring gene expression levels, the PCR method was used with proprietary primers and reagents from Novocasta and monoclonal antibody kits from Biosource (Belgium). The study of the drugs was conducted using an Olympus FluoView FV1000 confocal microscope, measuring the relative expression area in percentages.
In the second part of the study, we selected patients diagnosed with type 2 diabetes (n=36) and a group of patients with chronic pancreatitis in the remission phase (n=35). Clinically, we assessed the symptoms of well-being using the VAS scale, where 0 points indicate the worst well-being and 10 points the best. The parameters of the pancreas were also assessed using ultrasound data. The study was conducted on the high-precision GE Voluson E10 BT21 ultrasound system – set No. 4, manufactured by GE Healthcare (Austria), item number: VE10BT21-004.
We used German-made peptides IPH LGAT, which have all the necessary approvals and certifications for global markets, such as the WADA (anti-doping) certificate, MAFFA (safety) certificate, ORGANIC certificate, HALAL certificate, patent protection: US patent No. 5,405,266, European Union patent No. 016704471, Russian Federation patent No. 645608, and People’s Republic of China patent No. 30507522. The effectiveness of the IPH LGAT peptide was assessed by us before the start of the study and after 3 months.
When processing the study data, we calculated the mean intensive and extensive values with the calculation of the standard error; the significance of differences between two groups was evaluated using the Student’s t-test (a difference was considered significant at t>2, p<0.05).
Mutations in the genetic makeup of the pancreas lead to rapid development of glucose metabolism disorders and other consequences. Disruption of normal gene transcription results in early development of glucose tolerance disorders, type 1 and type 2 diabetes, and the formation of atypical tissues, both benign and malignant. Therefore, it is crucial to determine the possibility of influencing the genetic apparatus in terms of cytostatic preservation. To assess the cytostatic and oncoprotective properties of the IPH LGAT peptide in relation to the pancreas, we chose embryonic stem cells, which subsequently develop into organs and glands of the pancreas and other systems. The expression of BMP and FGF genes is responsible for the formation of the pancreatic enzyme apparatus, which later defines the signaling pathways for the formation of the pancreas itself. To determine the positive effect on the formation of normal pancreatic tissues, we studied the expression of BMP and FGF genes upon the application of the IPH LGAT peptide.
We decided to evaluate the expression of BMP and FGF genes, responsible for the formation of the pancreas and the synthesis of the enzymatic apparatus, to reveal the property of the IPH LGAT peptide regarding the normal formation of the pancreas (Figure 1).
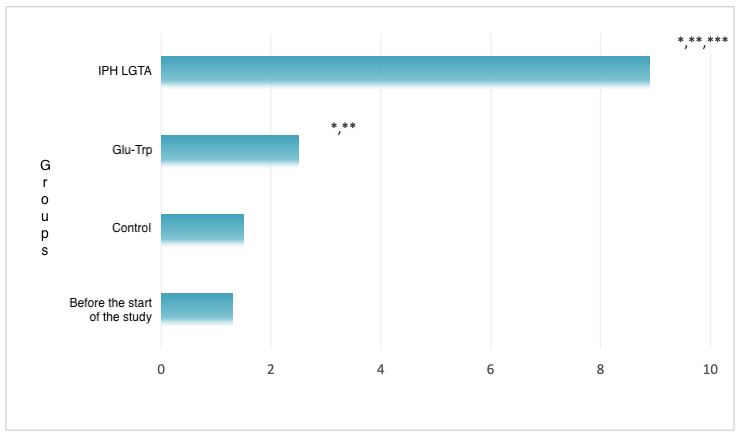
*p<0.05 compared to baseline data;
**p<0.05 compared to control;
***p<0.05 between the expression levels when applying Glu-Trp and IPH LGAT.
Figure 1. Expression of BMP and FGF genes.
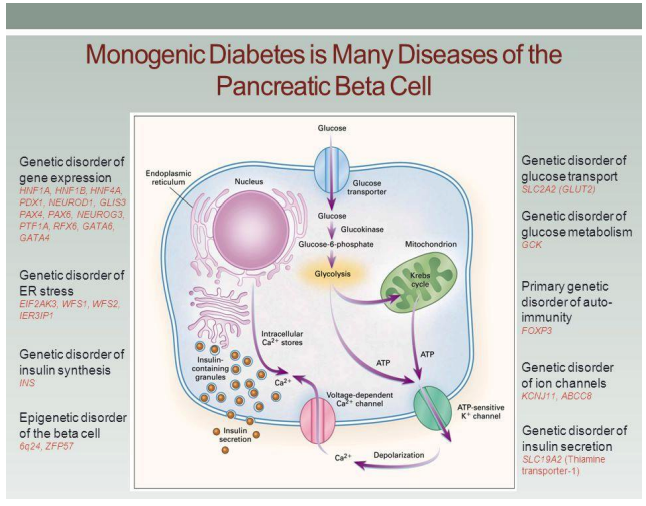
Figure 2. The influence of the PDX-1 gene on the formation of the pancreatic enzyme apparatus and the development of pancreatic pathology
We proved that after the application of the IPH LGAT peptide, the normal expression of BMP and FGF genes significantly increases by 82.3%, confirming the anti-tumor and cytostatic properties of the IPH LGAT peptide. This provides a basis to conclude that the application of the IPH LGAT peptide contributes to the normal and high-quality formation of the pancreas at the genetic level.
We also studied the influence of the IPH LGAT peptide application on the formation of normal secretory activity of the pancreas (Figure 2). The PDX-1 gene, one of the first discovered pancreas-specific transcription factors, is expressed by both dorsal and ventral pancreatic buds (Figure 3).
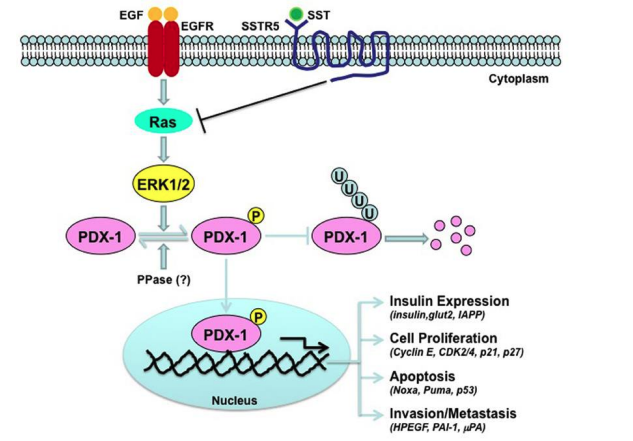
Figure 3. The role of the PDX-1 gene in regulating the formation of the pancreatic enzyme apparatus
Figure 3 shows that ablation of the PDX-1 gene leads to the cessation of gland development immediately after the formation of these structures. It has been found that mutation of the PDX-1 gene in humans causes agenesis of the pancreas. The function of PDX-1 is “educational”; besides, PDX-1 later participates in maintaining the identity of b-cells and the transcription of the insulin gene.
In our study, we proved that the application of the IPH LGAT peptide enhances the expression of the PDX-1 gene by 52.9%, which facilitates the formation of a normal pancreatic enzyme apparatus and is promising for use in predictive medicine programs in terms of preservation and restoration of pancreatic function (Figure 4).
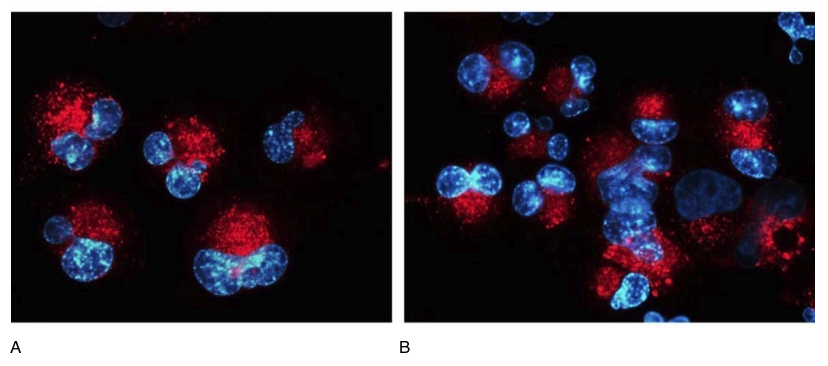
Figure 4. Expression of the PDX-1 gene (red fluorescent light, microscopy, 400×350)
A- without peptide application, B- with the application of the IPH LGAT peptide.
To further study the oncoprotective function of the peptide, we assessed the synthesis of the Ki67 protein, aggressive in terms of cancer development, both without peptide application and after the application of the IPH LGAT peptide. The Ki67 protein is also a known marker of cell proliferation during oxidative stress development. A decrease in the elevated expression of the Ki67 protein indicates the presence of antioxidant action and high regenerative capacity.
The experiment proved that the application of the IPH LGAT peptide reduces the expression of the Ki67 protein by 68.5%, as shown by microscopy in Figure 5. These data demonstrate the high oncoprotective and antioxidant function of the IPH LGAT peptide concerning the pancreas. The clinical efficacy of the IPH LGAT peptide application was studied by us through laboratory data, the perception of clinical symptomatology, and changes in pancreatic tissue according to ultrasound data in patients with chronic pancreatitis. C-peptide increases in the presence of type 2 diabetes (normal C-peptide levels – 0.9 – 7.1 ng/ml), glycated hemoglobin – one of the important control indicators of longitudinal sugar levels (normal glycated hemoglobin levels – 4.8 – 5.9 %), lipase increases in pancreatic disorders (normal lipase levels – 21 – 67 IU/l, chronic pancreatitis, pancreatic duct obstruction, secondary disorders in the presence of diabetes).
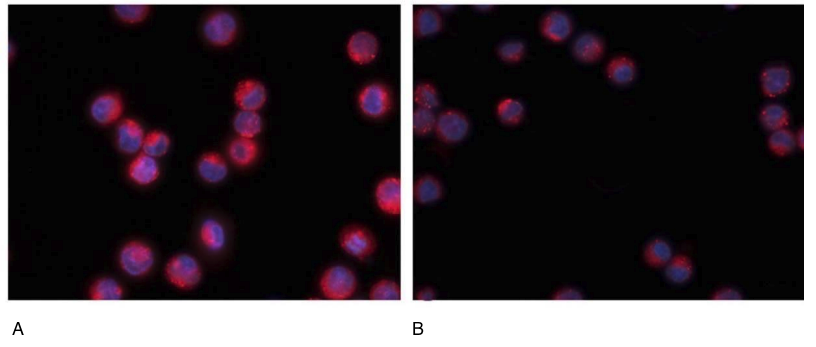
Figure 5. Expression of the Ki67 protein (red fluorescent light, microscopy, 400×350)
A- without peptide application, B- with the application of the IPH LGAT peptide.
Laboratory data are provided in Table 1. Thus, after the application of the IPH LGAT peptide, there was a significant decrease in C-peptide from 7.9 ng/ml to 6.2 ng/ml, normalization of glycated hemoglobin from 6.9% to 5.8%, and a decrease in lipase to mid-normal values from 65.9 IU/l to 47.7 IU/l. These data prove that the IPH LGAT peptide contributes to the normalization of pancreatic function in the presence of chronic pathology or not sharp deviations from the norm. Table 1 Laboratory data (M±m). *p<0.05, indicators before the application of the IPH LGAT peptide and after 3 months of application. Clinical symptomatology was studied in patients with compensated type 2 diabetes under therapy (Table 2). We found a significant improvement in the parameter “constant feeling of hunger” by 34.5%, “feeling of thirst” by 31.5%, “frequent urination” by 33.4%, “weakness, poor well-being” by 32.5%, “skin itching” by 35.2%.
Table 2 Clinical symptoms according to the VAS scale (points, M±m)
| Clinical Symptom | Before Study (Mean±SD) | After 3 Months of Use (Mean±SD) |
|---|---|---|
| Constant Hunger | 5.3±0.2 | 7.8±0.3* |
| Thirst Sensation | 5.2±0.2 | 8.1±0.3* |
| Frequent Urination | 5.1±0.2 | 8.6±0.3* |
| Weakness, Poor Well-being | 5.7±0.2 | 7.7±0.3* |
| Skin Itch | 5.3±0.2 | 8.2±0.3* |
*p<0.05, indicators before the application of the IPH LGAT peptide and after 3 months of application.
Such positive clinical dynamics increased the overall health and well-being indicator by 58.2%, as shown in Figure 6.
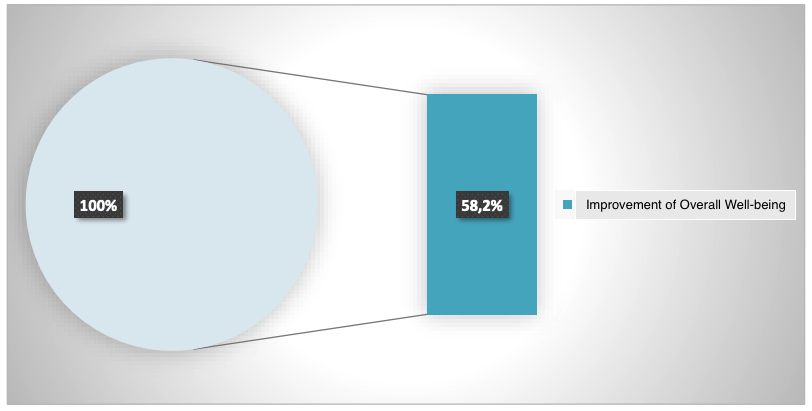
*p<0.05 between data before and after the application of the IPH LGAT peptide.
Figure 6. Indicator of improvement in overall well-being (%)
We also found that the application of the IPH LGAT peptide for 3 months reduces the CA 19-9 tumor marker level in patients with chronic pancreatitis by 57.8%, resulting in a decrease from 35.7 U/ml to 14.6 U/ml (Figure 7). The absence or low level of cancer antigen CA 19-9 in the blood is characteristic of healthy individuals. These data confirm the oncoprotective action of the IPH LGAT peptide. *p<0.05 between data before and after the application of the IPH LGAT peptide.
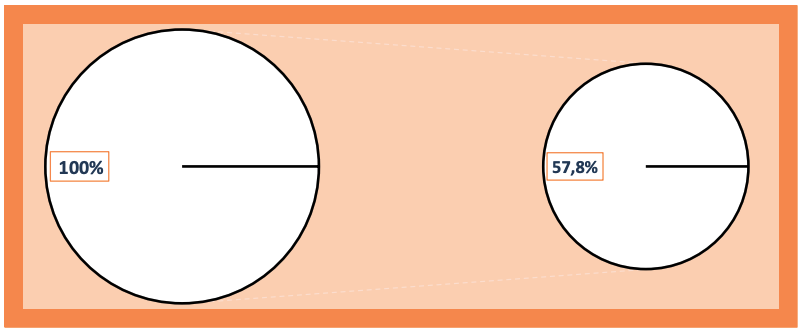
Figure 7. Dynamics of cancer antigen CA 19-9 in the blood after the application of the IPH LGAT peptide (%)
Next, we assessed changes in the pancreas in patients with chronic pancreatitis in remission. After the application of the IPH LGAT peptide for 3 months, there was a significant narrowing of the main pancreatic duct by 23.4%, a reduction in the volume of diffuse changes in the pancreatic parenchyma by 19.4%, and a reduction in size by 12.5% (Figure 8). These data indicate organ protection of the pancreas in patients with chronic pancreatic diseases. Thus, the application of the IPH LGAT peptide has oncoprotective and cytoprotective action, clinically improves overall well-being, and contributes to the functional stabilization of pancreatic function.
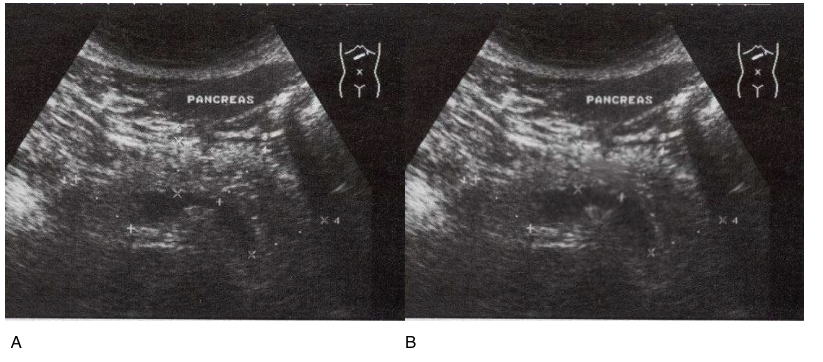
Figure 8. Ultrasound diagnosis of chronic pancreatitis in remission before and 3 months after the application of IPH LGAT in a 51-year-old patient
A- without peptide application, B- with the application of the IPH LGAT peptide.