
Thyroid hormones play a crucial role as regulators of growth, myelination of the nervous system, metabolism, and organ functions. Disorders affecting the thyroid gland are among the most common endocrinopathies in children, adults, and the elderly. Medical intervention requires an assessment of various characteristics of thyroid function and dysfunction across different ages. Early diagnosis and treatment are essential to prevent reversible and irreversible damage to the nervous system and a decline in quality of life. Therefore, in addition to examining the distinct features of disorders in hypothyroidism, hyperthyroidism, and normal thyroid function, it is promising to explore the expansion of possibilities for restoring thyroid function [1,2].
The development of thyroid neoplasms also warrants particular concern. In this context, therapeutic drugs with oncoprotective functions are considered interesting and relevant [3].
Additionally, iodine deficiency, smoking, and stress can also trigger hypofunction of the thyroid gland, and these factors accompany most people daily. Literature indicates that current smoking and a high degree of constant stress in population surveys are associated with a slight dose-dependent decrease in serum TSH levels, likely secondary to the increase in serum FT4 and FT3 levels caused by activation of the sympathetic nervous system. Smokers have an increased prevalence of nontoxic goiter and multinodular thyroid disease, at least in iodine-deficient areas. Current smoking reduces the dose-dependent risk of developing thyroid cancer, more pronounced for papillary than for follicular types, but these findings were not confirmed in subsequent studies. In an experimental animal model of autoimmune thyroiditis, there is evidence that nicotine has an anti-inflammatory effect. Conversely, smoking is a dose-dependent risk factor for the development of Graves’ hyperthyroidism and especially Graves’ ophthalmopathy. Smoking is associated with a higher frequency of Graves’ hyperthyroidism relapses and a higher risk of developing Graves’ ophthalmopathy [4,5,6].
Given the above, the study of the properties of drugs capable of preserving thyroid function is considered relevant.
This article will provide a brief overview of the results of a study on the effects of using the peptide IPH TR, which may contribute to optimizing thyroid gland function and, therefore, offer additional preventive benefits for overall health. The increase in levels of local and systemic biomarkers released upon peptide application and their protective nature underscores the importance of their use in preserving and restoring organ functions at any life stage and with any disease.
Objective: To explore the potential of the peptide complex IPH TR.
In the first part of our study, we conducted cell-based assays, selecting embryonic stem cells for their pluripotent nature, meaning they can differentiate into all three germ layers: ectoderm, endoderm, and mesoderm, which later develop into organs including the thyroid gland. We examined the expression of genes BRAF, KRAS, NRAS, HRAS, TERT, and the translocations RET/PTC, PAX8/PPARG using immunofluorescence (1:150, Abcam). The groups in this study included baseline molecular expression measurement, a control group (with added nutrient medium, incubated with serum albumin), a group with the control dipeptide Glu-Trp at 100 micrograms (µg), and a group with the peptide IPH TR at 100 µg. Gene expression levels were measured using PCR with primers and reagents from Novocasta and monoclonal antibody kits from Biosource (Belgium), analyzed with a confocal microscope Olympus FluoView FV1000, measuring relative expression area in percentages.
In the second part of the study, we selected patients diagnosed with thyroid cancer without metastases. Tumor and histologically altered thyroid tissue from consenting patients post-surgery were frozen and stored at -80°C. We assessed the expression of AKT/m-TOR pathway components (PTEN) in thyroid neoplasm tissues. PTEN concentration and purity were measured on a NanoDrop-2000 spectrophotometer (Thermo Scientific, USA), ranging from 80 to 250 ng/µl, with A260/A280 ratios between 1.95—2.05 and A260/A230 between 1.90—2.31.
Clinically, we evaluated symptom severity using the VAS scale, where 0 points indicate the worst possible well-being and 10 points the best. Thyroid nodule sizes were measured using the high-precision ultrasound system GE Voluson E10 BT21, manufactured by GE Healthcare (Austria), catalog number VE10BT21-004.
We used German-manufactured peptides IPH TR, which are approved and certified for global markets, including WADA (anti-doping), MAFFA (safety), ORGANIC, and HALAL certifications, and protected by patents in the United States (No. 5,405,266), European Union (No. 016704471), Russian Federation (No. 645608), and People’s Republic of China (No. 30507522). The effectiveness of the IPH TR peptide was evaluated before the study began and again after 3 months.
Data analysis involved calculating mean intensive and extensive values with standard error; differences between two groups were evaluated using Student’s t-test, with a difference considered significant at t>2, p<0.05.
Mutations in the genetic apparatus of the thyroid gland lead to the development of neoplasms. Accordingly, actions on the genetic apparatus from the perspective of onco- and cytoprotection can be beneficial for maintaining the proper functioning of the thyroid gland.
To assess the cytostatic and oncoprotective properties of the peptide IPH TR regarding the thyroid gland, we selected embryonic stem cells, which subsequently form the organs and glands of the thyroid and other systems. We decided to evaluate the expression of genes BRAF, KRAS, NRAS, HRAS, TERT, as well as translocations RET/PTC, PAX8/PPARG, responsible for the formation of the thyroid gland and synthesis of thyroid hormones, to identify the property of the peptide IPH TR regarding the function of the thyroid gland and its ability to normalize hormonal balance. The data are presented in Figure 1.
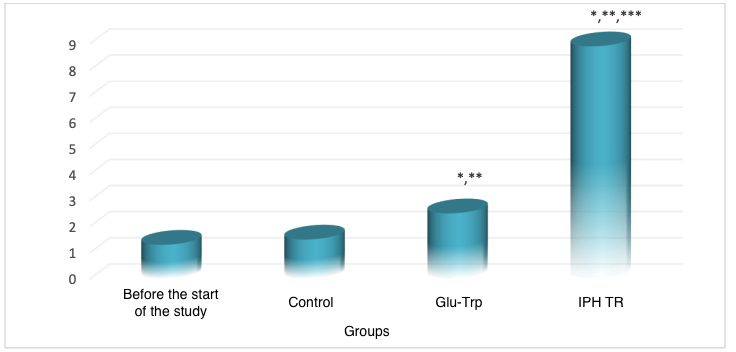
*p<0.05 compared to baseline data;
**p<0.05 compared to control;
***p<0.05 between expression levels when using Glu-Trp and IPH TR.
Figure 1. Expression of genes BRAF, KRAS, NRAS, HRAS, TERT, as well as translocations RET/PTC, PAX8/PPARG
Therefore, after the application of the peptide IPH TR, the normal expression of genes BRAF, KRAS, NRAS, HRAS, TERT, as well as translocations RET/PTC, PAX8/PPARG, increases by 71.9%, indicating the high oncoprotection and cytoprotection of the peptide IPH TR, which contributes to the normal formation of the hormonal background and the development of the thyroid gland.
Hyperactivation of the AKT/m-TOR signaling pathway is a characteristic feature of most cancer cells and apparently plays a key role in the mechanisms of tumor cell transformation and progression. A positive regulator of AKT/m-TOR pathway activity is the phosphatase PTEN, which acts as a tumor suppressor (Figure 2).
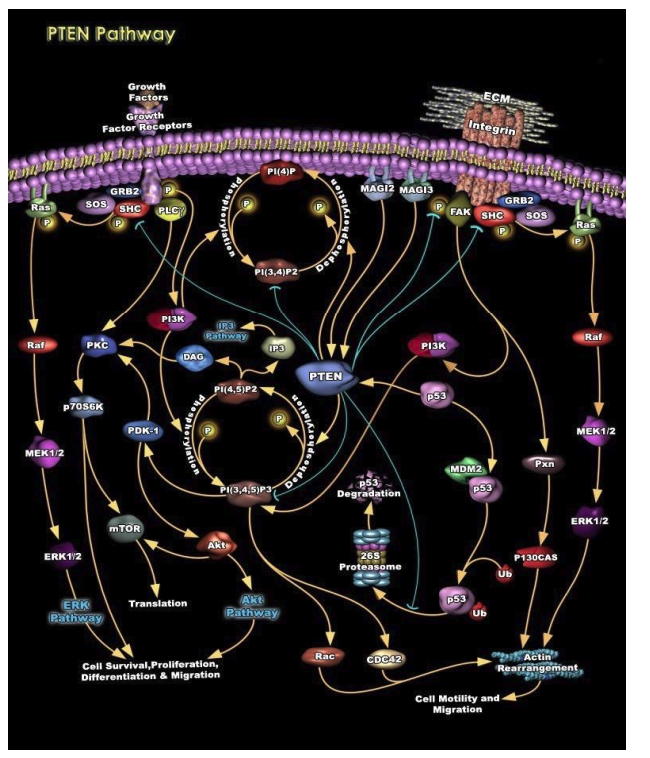
Figure 2. The role of phosphatase PTEN in regulating AKT/m-TOR signaling pathway activity
We observed a decrease in the phosphatase PTEN, which possesses anti-oncogenic activity, in cells of thyroid gland formation (thyroid cancer). We studied the effect of the peptide IPH TR on the synthesis of phosphatase PTEN (Figure 3).
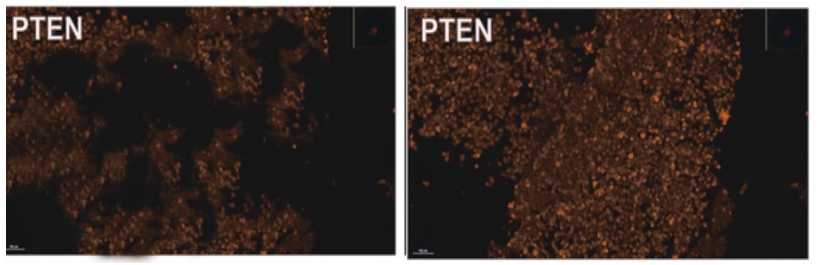
Figure 3. Expression of phosphatase PTEN (microscopy, 400x magnification)
It was found that the application of the peptide IPH TR increases the synthesis of phosphatase PTEN by 65.4%, which may be promising for the development of programs for preventing the development of thyroid cancer, particularly for preventing recurrences. At the same time, we noted the synthesis of highly differentiated cells of phosphatase PTEN, confirming the synthesis of high-quality molecules after the application of the peptide IPH TR.
An increase in the expression of the Ki67 protein is also one of the important mechanisms of oncopathology development. The application of the peptide IPH TR reduces the expression of the Ki67 protein by 84.6%, as shown in the microscopy results in Figure 4.
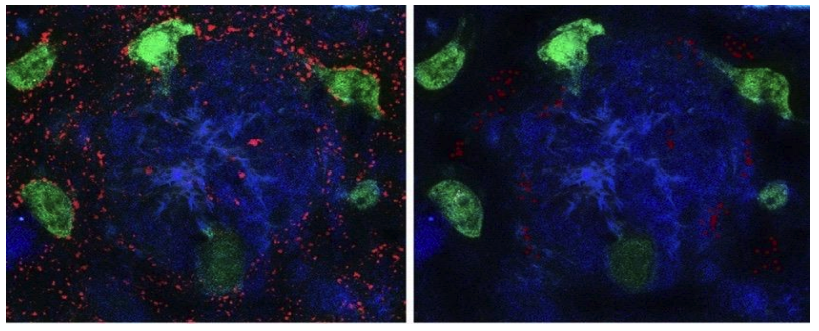
Figure 4. Expression of Ki67 protein (red fluorescent staining, microscopy, 400x magnification)
The clinical effectiveness of the peptide IPH TR application was studied by us based on the perception of clinical symptoms, blood tests, and the sizes of thyroid nodules in patients with diffuse euthyroid goiter (the stage of euthyroidism is either self-compensated or against the background of selected replacement therapy).
Table 1 Clinical symptoms according to the VAS scale (scores, M±m)
| linical Symptom | Before the Study | After 3 Months of Application |
|---|---|---|
| General Weakness, Increased Fatigue (average) | 5.7 ± 0.2 | 8.2 ± 0.3* |
| Headaches (average) | 5.4 ± 0.2 | 7.5 ± 0.3* |
| Dry Skin, Hair Loss, Decreased Body Temperature (average) | 4.9 ± 0.1 | 8.7 ± 0.3* |
*p<0.05, indicators before and after the application of peptide IPH TR for 3 months.
With the application of the peptide IPH TR in patients with diffuse euthyroid goiter, the sensation of weakness and increased fatigue decreased by 64.2%, the frequency of headaches decreased by 59.3%, and dry skin, hair loss, and decreased body temperature decreased by 69.4% (Table 1), which improved the overall health status and general well-being by 66.1%, as shown in Figure 5.
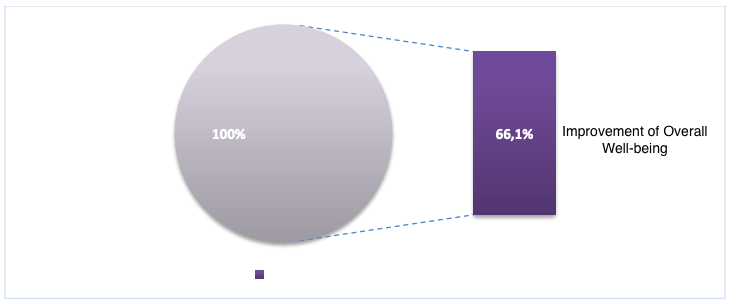
*p<0.05 between data before and after the application of peptide IPH TR.
Figure 5. Indicator of improvement in overall well-being (%)
After the application of peptide IPH TR for 3 months, a significant stabilization of nodule size was observed in 23.5% (from 2.5×1.2 to 1.9×1.0 cm), however, such a positive result was observed only in 33.5% of cases (Figure 6).
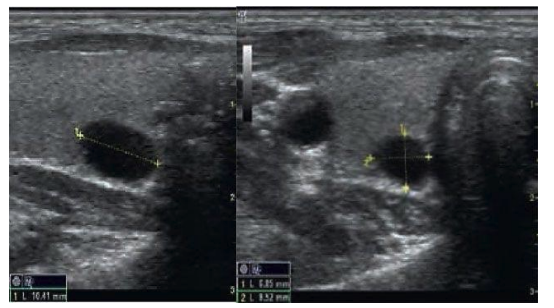
Figure 6. Ultrasound diagnostics in nodular euthyroid diffuse goiter before and after 3 months of IPH TR application in a 43-year-old patient
Thus, the application of the peptide IPH TR has oncoprotective and cytoprotective effects, clinically improves overall well-being, and contributes to the functional stabilization of thyroid function.
References