The liver is the primary organ that coordinates the metabolic flexibility of the entire body, which is characterized by the ability to dynamically adapt in response to fluctuations in energy needs. In this context, the mitochondria of hepatocytes are key partners in finely tuning metabolic flexibility. Increasingly, the literature is examining the metabolic and signaling pathways facilitated by mitochondria in the liver, the main pathways that regulate mitochondrial function, and how they operate in health and metabolic disorders associated with obesity, i.e., insulin resistance, non-alcoholic steatohepatitis, and hepatocellular carcinoma. Finally, strategies aimed at combating liver diseases to enhance overall human functional health are discussed [1,2].
Diffuse liver diseases have a diverse etiology, with each exhibiting characteristic morphometric changes. These changes are closely associated with intrahepatic hemodynamics at both the micro and macro levels, as well as specific pathophysiology. Short-term disturbances in intrahepatic hemodynamics, caused by each pathophysiological condition, are compensated by the balance of blood perfusion systems using potential transsinusoidal, transversal, and transplexal communication pathways (microhemodynamics), while long-term changes in intrahepatic hemodynamics lead to an increase in the overall resistance of liver vessels. Blood flow disturbances, caused by this increased vascular resistance, lead to necrosis and fibrosis of liver cells. These changes should be evenly distributed throughout the liver. However, morphometric changes occur unevenly: contraction or enlargement occurs unevenly. Against this background, a series of macro intrahepatic hemodynamic effects emerge, such as asymmetry and complication of liver morphometric structures, complex anatomy of the portal venous blood flow and hepatic venous outflow, as well as zonal differentiation between central and peripheral zones. These hemodynamic factors and pathophysiological changes are intricately linked to characteristic morphometric changes, based on a combination of selective atrophy and compensatory hypertrophy [3,4].
Imaging plays an important role in diagnosing and planning the treatment of patients with diffuse liver diseases. In certain diseases, such as disorders related to iron overload, fatty changes, hypothyroidism, diabetes, schistosomiasis, imaging results are characteristic and diagnostic. In other cases, the results are less specific, but imaging remains useful for assessing changes related to fibrosis, cirrhosis of the liver, and portal hypertension. In any case, familiarity with these diffuse liver diseases and the expected imaging outcomes allows for an organized and thoughtful evaluation, paying close attention to key diagnostic features and important implications [5].
In light of the above, the study of the properties of drugs capable of preserving liver function is considered relevant. This article will present a brief overview of the research results on the effects of using the peptide IPH LIV, which may contribute to optimizing liver function and, therefore, offer additional preventive benefits for overall health status. The increase in levels of local and systemic biomarkers, which are released upon peptide application and possess protective qualities, indicates that their use is important in terms of preserving and restoring organ functions at any life stage and under any disease conditions.
Objective: To explore the possibilities of the peptide complex IPH LIV.
In the first part of the study, we conducted tests on cell materials. We chose stem cells for this purpose. We examined the expression of the SERPINA1 gene, which codes for A1AT – G264V, and G342L. The groups for the study were: measuring molecule expression before the start of the study, control (adding nutrient medium, incubating with serum albumin), adding control dipeptide Glu-Trp at a concentration of 100 micrograms (μg); adding peptide IPH LIV at a concentration of 100 micrograms (μg). The PCR method was used. The study of the drugs was conducted using the Olympus FluoView FV1000 confocal microscope, measuring the relative area of expression in %.
In the second part of the study, we selected patients (main group, group 1) who had a conclusion from ultrasound diagnostics – diffuse liver changes and normal/increased levels of ALT, AST (n=30 people, average age 41.3±1.2 years), who were subjected to a comprehensive program using hepatoprotectors – peptides IPH LIV, and a group of patients (control group, group 2) with the same laboratory and instrumental studies (n=32 people, average age 41.3±1.2 years), but who underwent a traditional treatment and patient management program. Patients were relatively healthy, did not have acute diseases or diseases in the decompensation stage, and did not take medication that could distort the results.
The condition of the liver was assessed by the level of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin, alkaline phosphatase (ALP) in the blood biochemical analysis. The following levels were considered normal: ALT – 7.0–41.0 U/L, AST – 10.0–40.0 U/L, total bilirubin – 3.4–18.8 µmol/L, ALP – 36-105 U/L. Examinations were conducted at the initial patient consultation. All patients underwent an ultrasound examination of the liver, performed by a diagnostician with over five years of experience on the Siemens Acuson P500 expert-class ultrasound scanner (Germany, article M01706). The multiparametric ultrasound examination of the liver was performed using a convex probe at a frequency of 6 MHz in B-mode, color and pulse-wave Doppler modes, and shear wave elastography. The examination was conducted with the patient “lying on their back” with free breathing, the right hand behind the head. In B-mode, the sizes of the right and left liver lobes (within the age norm, enlarged), echogenicity (isoechoic, hypoechoic, hyperechoic), and echostructure (homogeneous, heterogeneous) were assessed, the presence of sediment in the gallbladder, the condition of intrahepatic ducts, and the sizes of the spleen were evaluated. The portal vein was assessed in B-mode and color Doppler with measurement of its diameter around the liver gate in mm (normally up to 13 mm), after which the blood flow speed in it in cm/s (normal values 16-40 cm/s) and the type of blood flow (usually hepatopetal) were determined. The study of the inferior vena cava was conducted with incomplete breath-hold on inspiration, determining the type of blood flow (phasic, antegrade) and its diameter (normally – up to 20 mm). In pulse-wave Doppler, the resistance index of the hepatic artery was determined (normally 0.55–0.7). Ultrasound signs of hepatobiliary system changes in B-mode were consistent with the following parameters: enlargement of the right (more than 14 cm) and left (more than 6 cm) liver lobes, increased echogenicity and heterogeneity of the parenchyma, portal vein diameter more than 13 mm and inferior vena cava more than 20 mm, presence of sediment in the gallbladder, increased diameter of the common bile duct (more than 6 mm), compression of intrahepatic bile ducts, increased spleen sizes (longitudinal size more than 12 cm and transverse size more than 6 cm), growth of the spleen vein diameter (more than 7 mm), presence of free fluid. During color and pulse-wave Doppler mapping, changes in blood supply were established with a blood flow speed in the portal vein below 16 cm/s, the appearance of hepatofugal blood flow type in the portal vein, and pulsating type of blood flow in the inferior vena cava, increase in the resistance index in the hepatic artery above 0.7.
Liver elastography with shear wave was conducted using a curvilinear probe with intercostal access, placing the central beam perpendicular to the liver capsule. Measurements of liver parenchyma stiffness in kPa were conducted in the VirtualTouch program, with the area of interest defined in regions without large vessels and away from the gallbladder. Indicators were determined in ten points of the right lobe at a depth of 3-8 cm from the skin surface, with subsequent determination of the average indicator. The degree of liver fibrosis was determined according to the METAVIR scale, according to which the degrees of fibrosis corresponded to the following liver stiffness indicators: F0 (no fibrosis) ≤6.0 kPa, F1 (mild fibrosis) – from 6.0 to 7.0 kPa, F2 (moderate fibrosis) – from 7.0 to 9.5 kPa, F3 (severe fibrosis) – from 9.5 to 12.5 kPa, F4 (cirrhosis) >12.5 kPa.
Clinically, we assessed the symptomatology of well-being on the VAS scale, where 0 points – the worst feeling, 10 points – the best feeling.
We used German peptides IPH LIV, which have all permits and approvals for global markets, such as: WADA certificate (anti-doping), MAFFA certificate (safety), ORGANIC certificate, HALAL certificate, patent protection: patent in the United States of America No. 5,405,266, patent in the European Union No. 016704471, patent in the Russian Federation No. 645608, patent in the People’s Republic of China No. 30507522. The effectiveness of the use of peptide IPH LIV was assessed by us before the study and after 3 months.
When processing the study data, an average of intensive and extensive quantities was calculated with the determination of the standard error; the significance of differences between two populations was assessed using the Student’s t-test (a difference in indicators was considered significant at t>2, p<0.05).
Mutations in the liver’s genetic apparatus lead to rapid development of metabolic disorders. To evaluate the cytostatic and oncoprotective properties of the peptide IPH LIV with respect to the liver, we selected embryonic stem cells, from which the liver subsequently forms. We studied the expression of the SERPINA1 gene, encoding A1AT – G264V and G342L, which is responsible for liver formation (Figure 1).
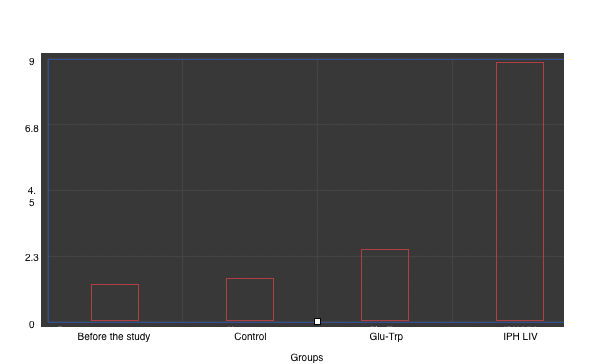
*p<0.05 compared to baseline data;
**p<0.05 compared to control;
***p<0.05 between the expression level indicators when applying Glu-Trp and IPH LIV.
Figure 1. Expression of the SERPINA1 gene, encoding A1AT – G264V and G342L.
The expression of the liver formation gene significantly increases by 79.6%, confirming the antitumor and cytostatic properties of the peptide IPH LIV. This gives reason to conclude that the application of the peptide IPH LIV contributes to normal and quality liver formation at the genetic level.
However, to verify the antitumor activity effect, we studied the expression of the Ki67 neoplasm protein against the background of applying the peptide IPH LIV. The decrease in Ki67 protein expression by 89.6% after the application of the peptide IPH LIV confirms the antitumor action and can lead to an increase in organism functioning and survival, which is due to the dynamics of Ki67 protein production in the body (Figure 2).
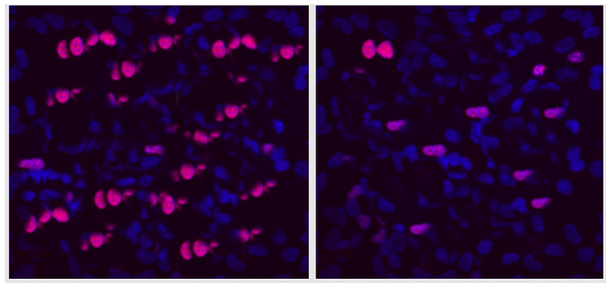
A B
Figure 2. Expression of Ki67 protein (red fluorescent glow, microscopy, 400×350).
A- without peptide application, B- with the application of the peptide IPH LIV.
The conduct of laboratory instrumental studies revealed the following changes after 3 months of observation. An increase in ALT levels was observed in 25 (78%) patients of the second group and 18 (60%) of the first group, an increase in the concentration of drugs was observed in 15 (47%) and 9 patients (30%), respectively, total bilirubin – in 11 (34%) and 4 patients (13%), respectively. The statistically processed test indicators are presented in Table 1.
Table 1. Laboratory data
| Laboratory Data | 1st Group, Main, Application of Peptide IPH LIV | 2nd Group, Control, Without Peptide IPH LIV |
|---|---|---|
| ALT (U/L) (10-35 U/L) | 28.9 [21.2;33.2] | 37.2 [31.1;42.3]* |
| GGT (U/L) (6-49 U/L) | 42.7 [36.4;46.1] | 51.2 [46.1;59.1]* |
| Drug Concentration (%) | 24.3±0.8 | 38.2±1.2* |
*p<0.05 compared to the indicators after applying the peptide IPH LIV.
Therefore, the application of the peptide IPH LIV improves the liver condition by an average of 46.7% according to liver enzyme levels, proving the hepatoprotective properties of the peptide.
Clinical symptoms of the disease were more common in patients from the second group than in the first. In percentage terms, heaviness in the right hypochondrium was detected in 90.9% and 67.8% of patients, respectively, skin itching – in 61.2% and 48.7%, diarrhea – in 81.2% and 63.4%, constipation – in 89.5% and 79.6%, bloating – in 81.3% and 62.4%. There was a positive clinical state dynamics after applying the peptide IPH LIV (Table 2), confirming the capabilities of the peptide IPH LIV as a hepatoprotector – the basis of physical functioning of the body.
Table 2. Clinical symptoms on the VAS scale (points, M±m)
| Clinical Symptom | 1st Group, Main, Application of Peptide IPH LN | 2nd Group, Control, Without Application of Peptide IPH LN |
|---|---|---|
| Heaviness in the right hypochondrium | 8.2±0.2 | 5.8±0.1* |
| Skin itching | 7.9±0.2 | 5.1±0.1* |
| Diarrhea | 8.1±0.2 | 5.6±0.1* |
| Constipation | 8.7±0.2 | 5.7±0.1* |
| Bloating | 7.3±0.2 |
*p<0.05 compared to the indicators after applying the peptide IPH LIV. Such positive clinical dynamics increased the physical health indicator and overall well-being by 26.1% and 35.2% after applying the peptide IPH LIV, as shown in Figure 3.
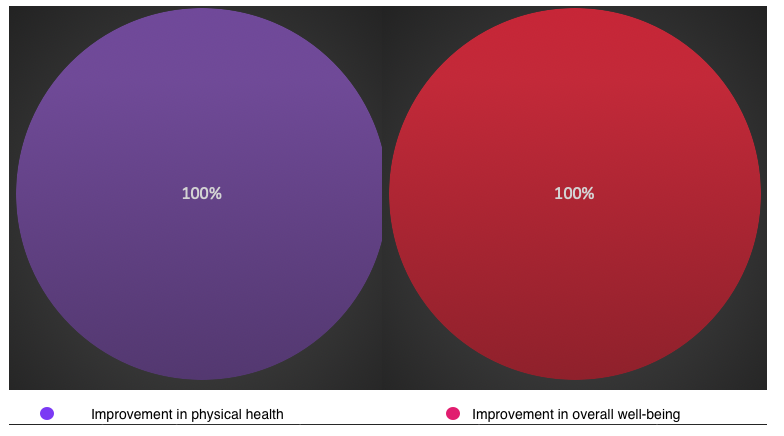
*p<0.05 compared to the indicators after applying the peptide IPH LIV.
Figure 3. Health status dynamics (%).
It was also found that the application of the peptide IPH LIV reduces the alpha-fetoprotein (AFP) tumor marker indicator by 47.6% (Figure 4). These data confirm the oncoprotective action of the peptide IPH LIV.
*p<0.05 compared to the indicators after applying the peptide IPH LIV.
Figure 4. AFP tumor marker synthesis indicator (%).
Analysis of changes in ultrasound signs of hepatobiliary system damage in patients of both groups showed that all the above deviations were more often observed in the 2nd group. According to the ultrasound picture, a small number of patients from the first group after applying the peptide IPH LIV showed changes such as an increase in liver size, increased echogenicity, heterogeneity of echostructure, and an enlarged spleen. These changes were observed even more frequently in patients from the 2nd group without applying the peptide IPH LIV. Other indicators in B-mode in patients from the 1st group after applying the peptide IPH LIV were within the norm, while in those examined from the 2nd group without applying the peptide IPH LIV, an expansion of the portal vein (40%) and inferior vena cava (16%) was observed. During dopplerography, no deviations in the blood flow of the liver vessels were detected in patients from the 1st group after applying the peptide IPH LIV, while in some patients from the 2nd group without applying the peptide IPH LIV, an increase in blood flow speed in the portal vein, an increase in the resistance index in the hepatic artery, and the appearance of pulsating blood flow in the inferior vena cava were observed. Also, in patients from the 1st group after applying the peptide IPH LIV, mainly fibrosis of F1 degree was observed, and in patients from the 2nd group without applying the peptide IPH LIV – F1, F2, F3 degrees. In 24 patients from the 1st group after applying the peptide IPH LIV with fibrosis of F0 degree, the average liver stiffness was 4.56±1.17 kPa, in 6 patients with fibrosis of F1 this indicator was 6.62±0.49 kPa, in 2 patients with fibrosis of F2 stiffness was equal to 8.5 kPa and 8.9 kPa, respectively. In patients from the second group without applying the peptide IPH LIV with a fibrosis degree of F0, the average liver stiffness was 5.2±0.51 kPa, with F1 – 6.6±0.56 kPa, with F2 – 8.5±0.64 kPa, with F3 – 10.1±0.46 kPa. Increased liver stiffness has a strong correlation with worsening clinical symptomatology (r=0.993, p<0.05). The obtained data confirm the reduction of liver stiffness after applying the peptide IPH LIV (Figure 5).
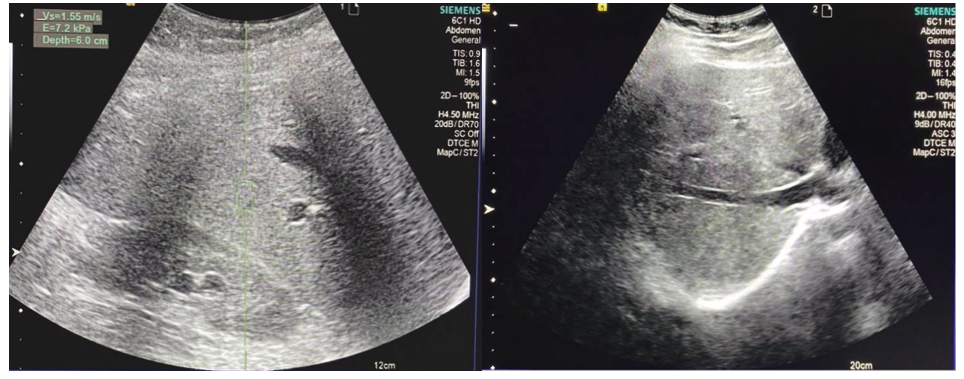
A B
Figure 5. Liver stiffness indicator. A- without applying the peptide IPH LIV, B- with the application of the peptide IPH LIV. These data testify to liver organoprotection in patients with diffuse liver changes.
Thus, the application of the peptide IPH LIV has oncoprotective and hepatoprotective actions, clinically provides an improvement in overall well-being and contributes to the enhancement of physical functioning of the body.