
The article describes the result of biological and clinical protective effects of the peptide IPH AGAA. The peptide IPH AGAA is a high biological activity to the control the normal formation of muscle system. The application of the peptide IPH AGAA regulates metabolism in muscle tissue and the level of development of speed-power qualities in humans, which also allows to optimize the metabolism in muscle cells and provide antioxidant action, physical activity to prevent damage to muscle cells by free radicals. The application of the peptide IPH AGAA leads to optimization of metabolism in muscle cells, improvement of microcirculation in muscle tissue and restoration of water and mineral balance in muscles. The peptide IPH AGAA has anti-inflammatory action against muscle tissue damage and thus contributes to the improvement of regeneration of damaged muscles, which is manifested by cytostatic and anti-inflammatory action against muscle cells according to experimental studies. The application of the peptide IPH AGAA optimizes metabolism in muscle cells, has an antioxidant effect, prevents damage to muscle cells by free radicals, provides intensive and long-term nutrition of muscle cells, has a stimulating effect on muscles in hypoxia and increases the elasticity of muscles. According to clinical data, the application of the peptide IPH AGAA optimizes metabolism in muscle cells, improves microcirculation in muscle tissue, provides intensive and long-term nutrition of muscle cells, has a stimulating effect on muscles in hypoxia and increases the elasticity of muscles. The application of peptide IPH AGAA recommended in the form of biologically active food supplement with therapeutic and preventive purposes for the normalization of the system functions of muscles.
The study of the effects of peptides has a great interest today. Peptides have the same structure as proteins, but the size of these molecules is smaller. It is also important that short peptides are a natural metabolic product in the body and they can`t be detected in blood or urine. That`s why, it is interesting to study the effects of peptides on cell cultures [1, 2, 5].
The peptide IPH AGAA contains a low molecular weight peptide, it has myoprotective properties and has a normalizing effect on muscle tissue [3, 4, 6].
The results of experimental studies have shown that the peptide IPH AGAA regulates metabolic processes in myocytes, increases the reserve capacity of the body, which suggests the effectiveness of the peptide IPH AGAA to normalize the functions of the human muscular system in disorders of various origins [1, 7].
The aim of this study was to identify biological and clinical myoprotective effects of the peptide IPH AGAA.
Material and methods.
We conducted 3 areas of research to identify the biological and clinical effects of the peptide IPH AGAA:
In a cell-based study we selected embryonic stem cells to study the cytostatic, geroprotective and oncoprotective properties of the peptide IPH AGAA in relation to the muscle tissue. These cells belong to the pluripotent type, which means that they can be differentiated into all three primary germ sheets: ectoderm, endoderm and mesoderm. Organs and glands of the muscle tissue and other systems are formed from these primary germ sheets in the future. The human embryo transformes the blastocyst stage in 5-6 days after fertilization. The stem cells are obtained from the blastocyst.
The genes responsible for muscle tissue formation are the ACTN3 gene and the MSTN gene. The ACTN3 gene encodes the protein alpha-actinin-3 (ACTN3), which stabilizes the contractile apparatus of skeletal muscles and participates in a large number of metabolic processes. The localization of the gene on the chromosome 11q13.2. Polymorphism of the ACTN3 gene is one of the causes of changes in metabolism in muscle tissue and reduce the level of development of speed-power qualities in humans. In humans, myostatin is encoded in the MSTN gene encoded by myostatin. Myostatin (growth and differentiation factor 8) is a protein that inhibits the growth and differentiation of muscle tissue. The experimental studies show that blocking the action of myostatin leads to a significant increase in dry muscle mass with almost complete absence of adipose tissue. This usually limits muscle growth, ensuring the absence of hypertrophied muscle tissue. In connection with these data, we decided to study the activity of this gene in the application of the peptide IPH AGAA.
We also assessed the biological active markers. We used immunofluorescence technique using primary antibodies to Mif5 (1:150, Abcam), ACTN3 (1:250, Abcam) and p53 protein (1:50, Abcam).
We have created the following groups for the study: 1 group – the study of molecular expression before the study; 2 group-control (we added the culture medium, incubation with serum albumin); 3 group – we added the control dipeptide Glu-Trp at the concentration of 100 micrograms (mcg); 4 group – we added the peptide IPH AGAA at a concentration of 100 micrograms (mcg). We selected the peptide Glu-Trp with the immune properties and well described in the literature as a control.
The PCR method was used to measure the level of gene expression using Novocasta’s reagents and sets of monoclonal antibodies produced by Biosource (Belgium). We used confocal microscope Olympus FluoView FV1000 with indicator of 200, 400, 600. We conducted the measurement of the expression in %.
We have chosen the most commonly used species of laboratory animals for the study for the experiment recommended by the Ministry of Health of the Russian Federation in the Manual for preclinical studies of drugs – rats.
We created an experimental model of muscle injury. In order to cause muscle injury, we introduced the drug nortexin into the quadriceps of the left limb of rats. We studied 40 rats aged 14.1±1.2 months and weighing 408.9±8.9 g, which created the conditions of muscle injury. All procedures of animal keeping and testing were carried out in accordance with standards ISO 10993-1- 2003 and GOST RISO 10993.2-2006. The rats were divided into 2 groups – the control (n=20) and the main group (n=20). The rats of the main group were given orally through a pipette-dispenser a solution consisting of water for injection in a dosage of 1 ml, in which the lyophilized powder of IPH AGAA peptides was dissolved in a concentration of 0.59 micrograms (mcg) per rat body weight per day for 14 days. A pipette-dispenser allowed to control the volume and the fact of liquid consumption. On day 13 venous blood sampling was performed and the level of proinflammatory (TNFα, IL-1-β) and antiinflammatory (Il-10) cytokines was investigated. The determination of the level of cytokines TNF-α, IL-1-β, IL-10 was carried out on the automatic biochemical and enzyme immunoassay
ChemWell 2910 (Combi) (Awareness Technology, Inc., USA.)
The rats were killed after 14 days. Then the quadriceps muscle of the left limb was removed, fixed by immersion in a solution of 4% paraformaldehyde in phosphate buffer (PBS pH = 7.3) for 24 hours at a temperature of 4 °C. We produced slices with a thickness of 20 µm using cryotome of Leica CM 1510S model (Germany). Then the sections were mounted on a slide and stained with hematoxilin and eosin. We used the Olympus IX81 microscope for the study. The Danet criterion was used to assess the reliability of the difference in the results obtained in the groups before the use of the peptide, compared with the groups after the application of the peptide IPH AGAA.
The clinical studies of the peptide IPH AGAA were conducted in 77 men aged 30 to 58 years (mean age was 41.1±1.2 years). Inclusion criteria: patients with compensated pathologies. Exclusion criteria: acute diseases, patients with decompensated pathology, patients with exacerbation of chronic diseases.
We conducted studies the effectiveness of peptides in the dosage of 50 µg (n= 75 people) and 150 µg (n= 76 people) to assess the effectiveness of the dose of 100 µg (n=77 people) for the peptide IPH AGAA. The peptide IPH AGAA was administered orally: 1 capsule (100 µg peptide) 1 time per day for 30 days, then 30 days a break in the medication. And repeat the same course for another 30 days, again 30 days a break in the medication – and the third course for 30 days. The total course was 6 months (3 courses of 30 days and 3 a break in the medication of 30 days). We studied the effectiveness of the improved management scheme of such patients using the peptide IPH AGAA after 3 and 6 months. The control values was selected the results before the study.
The effectiveness of the peptide IPH AGAA was evaluated on the basis of subjective sensations (using VAS – visual analog scale), muscle mass dynamics (using the Body composition monitor OMRON BF508, manufacturer: Omron (Japan), model: HBF-508-E) and dynamometry (using the dynamometer Megaton ADC/6P-1000/6I-1). The relative strength index (SI) was calculated using the formula: SI= strength of the strongest hand/ body weight x 100%. The norm for men is 65-80%.
We used standard statistical methods of medical and biological research.
Results and discussion.
The biological analysis of myoprotective effects of the peptide IPH AGAA on cell culture
In figure 1, we show that the peptide IPH AGAA significantly increases the expression of the gene ACTN3, responsible for the normal formation and formation of the muscle system, in particular, regulating metabolism in
muscle tissue and the level of development of speed-strength in humans, which also allows to optimize the metabolism in muscle cells and provide antioxidant action, with exercise to prevent damage to muscle cell by free radicals.
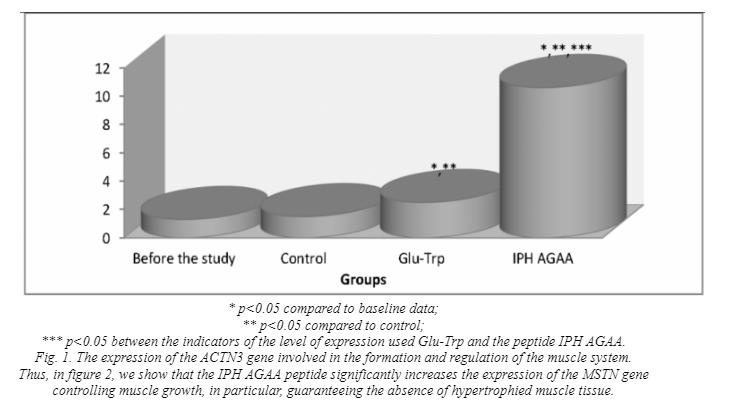
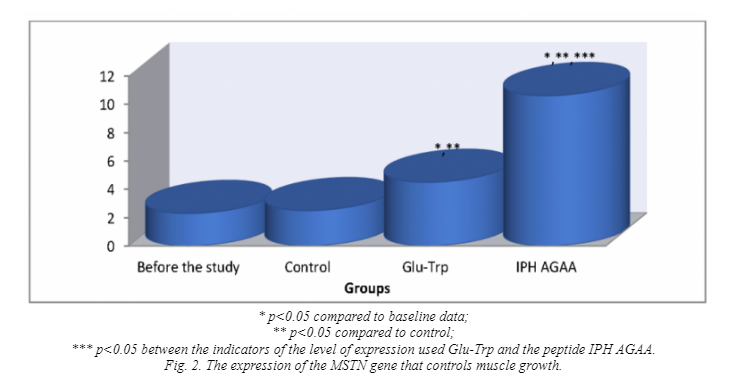
Thus, from the above data it can be seen that under the influence of the peptide IPH AGAA in human cell culture there is a statistically significant increase in the expression of genes responsible for the ontogenesis of the muscular system. These data show that the peptide IPH AGAA significantly increases in the culture of human cells “cascade” of signal molecules, which is necessary to activate the processes of proliferation and differentiation of stem cells in the cells of the muscular system, the formation of muscle system, regulation of metabolism in muscle tissue and thus determine the level of development of speed-strength in humans.
Figure 3 shows that the application of the peptide IPH AGAA increases the expression of Mif5 in 4 times from the baseline, which is a key transcriptional factor in the regulation of skeletal muscle myogenesis.
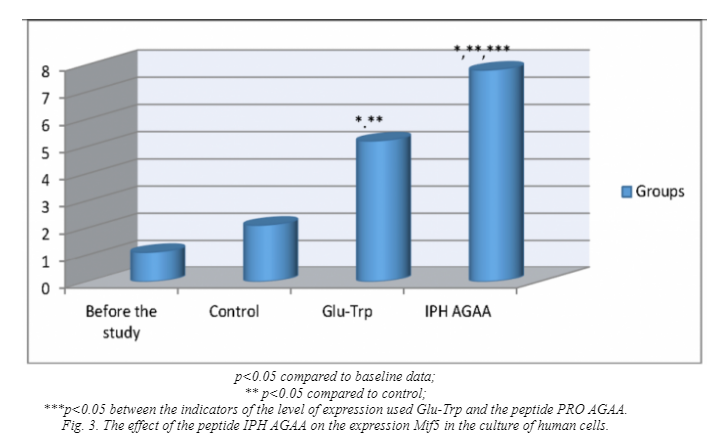
Thus, the application of peptide IPH AGAA induces myogenic differentiation of pluripotent cells towards the normal formation of skeletal muscle and provides intense and long lasting nourishment to the cells of the muscle tissue.
Figure 4 shows that the use of IPH AGAA peptide significantly increases the expression of ACTN3 protein in 4.5 times from the initial level, which stabilizes the contractile apparatus of skeletal muscles and participates in a large number of metabolic processes, which leads to the optimization of metabolism in muscle cells, improve microcirculation in muscle tissue and restore water and mineral balance in muscles.
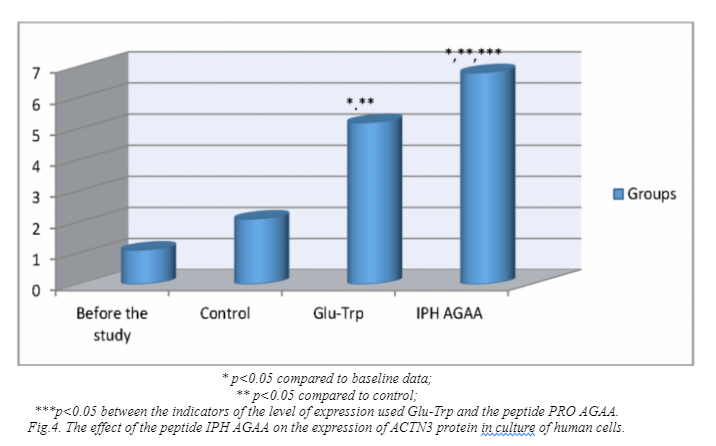
Thus, the application of the peptide IPH AGAA has a pronounced myoprotective nature, in particular, increasing the activity of actin fibers in the muscle, increasing the contractility and strength of muscle tissue, thereby increasing the elasticity of the muscles and providing a stimulating effect on the muscles in hypoxia.
The effect of the peptide IPH AGAA on the expression of p53 protein in cell cultures is presented in figure 5. The application of the peptide IPH AGAA increases the production of protein p53, which is a transcriptional factor and acts as a suppressor of malignant tumor formation by the way of activating apoptosis in the tissues. This results lead to the conclusion about the antitumor properties of the peptide IPH AGAA.
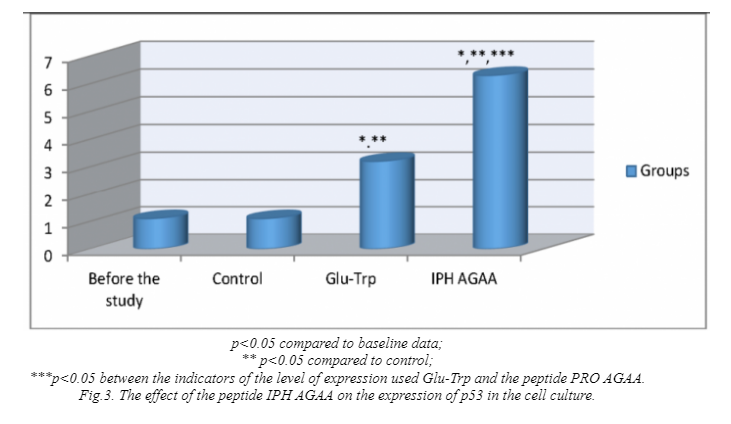
P53-dependent apoptosis also avoids the accumulation of mutations. In the case when mutations have already arisen, p53-dependent apoptosis allows to eliminate this potentially dangerous cells. On this fact we can make a conclusion about the cytoprotective effect of the peptide IPH AGAA.
The peptide IPH AGAA had a high onco-protective activity in relation to the cells of the muscle system according to the expression of biological molecules in cell culture.
Biological analysis of myoprotective effects of the peptide IPH AGAA in an experimental model
During the experiment, it was shown that the application of the peptide IPH AGAA significantly positively affects the reduction of chronic immune inflammation, developed in response to muscle injury, and increased anti-inflammatory response. The data are given in table 1.
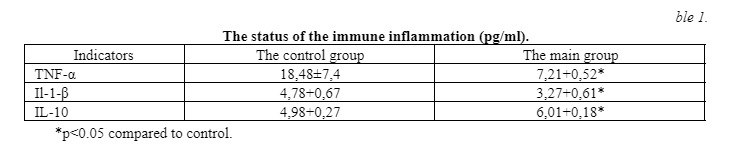
These data allow us to conclude that the IPH AGAA peptide has anti-inflammatory action against muscle tissue damage and thus improves the regeneration of damaged muscles.
During the experiment, we found that in the control group in the section of the quadriceps muscle in the control group, there were insignificant areas of fiber structuring, striation, which amounted to an area of muscle fiber restoration of only 36.8±1.2% and indicates a high risk of contractures and a decrease in the elasticity of the muscles. While in rats injected with the peptide IPH AGAA, fiber structure was observed, the striation and muscle fiber recovery area was 89.4±1.4%, which is in 2.4 times more than without the application of the peptide IPH AGAA (figure 6).
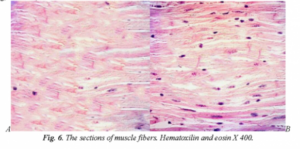
A – without the application of peptide IPH AGAA.
B – with the application of peptide IPH AGAA.
Thus, the application of the peptide IPH AGAA reduces the degree of inflammation in muscle tissue damage, improves regeneration and restoration of muscle fibers, prevents the development of contractures and reduce muscle elasticity in the experimental model. These data indicate that the application of the peptide IPH AGAA optimizes metabolism in muscle cells, has an antioxidant effect, prevents damage to muscle cells by free radicals during physical activity, provides intensive and long-term nutrition of muscle cells, has a stimulating effect on muscles in hypoxia and increases the elasticity of muscles.
Clinical analysis of myoprotective effects of the peptide IPH AGAA
The results of a clinical study of the application of the peptide IPH AGAA showed that in 3 months after the application of this peptide, the subjective assessment of physical activity, energy and endurance is significantly improved in 1.5 times compared with the initial data (table 2).
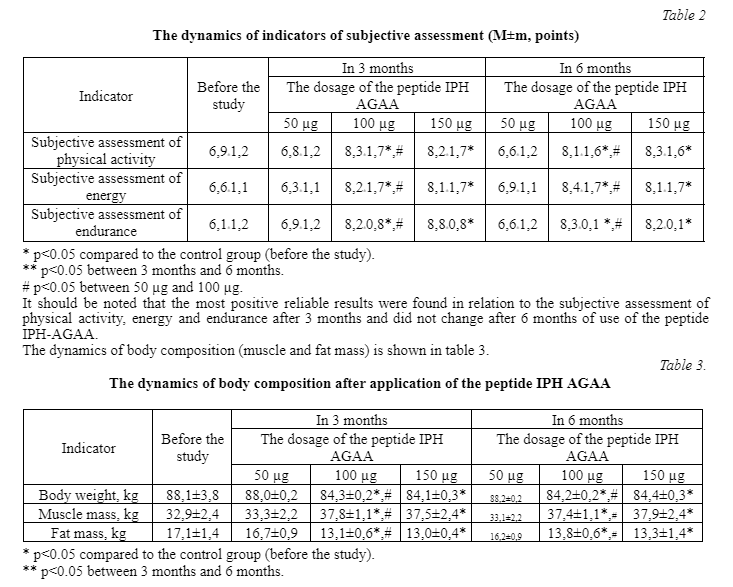
# p < 0.05 between 50 µg and 100 µg. Attention is drawn to the fact of a significant decrease in total weight, increase in muscle mass in 1.1 times and a decrease in fat mass in 1.3 times after the application of the peptide IPH AGAA compared to the baseline, p < 0.05 between the indicators compared with the control group (before the study), p 0.05 between the indicators after 6 months and 3 months, which indicates the properties of this peptide in terms of normalization of muscle tissue functions and redistribution of muscle mass, that is, a change in body composition. It should be noted that the application of the peptide IPH AGAA significantly improves the performance of dynamometry in 1.2 times compared to the original data. Indicators after 3 months of the application of the peptide IPH AGAA reach the upper limit of the norm. While the baseline refers to a lack of muscle strength (below the lower limit of normal). The data are given in table 4.
The performed studies confirm the high biological activity of the peptide IPH AGAA in relation to the control of the normal formation of the muscle system in humans at the genetic level according to the expression of genes responsible for the ontogenesis of the muscular system. In particular, these data confirm that the application of the peptide IPH AGAA regulates metabolism in muscle tissue and the level of development of speed-power qualities in humans, which also allows to optimize the metabolism in muscle cells and provide antioxidant action, physical activity to prevent damage to muscle cells by free radicals. The peptide IPH AGAA significantly increases in human cell culture “cascade” of signal molecules, which is necessary to activate the processes of proliferation and differentiation of stem cells in the cells of the muscular system, the formation of muscle system, regulation of metabolism in muscle tissue and thus determine the level of development of speed-power qualities in humans. The application of the peptide IPH AGAA induces differentiation of polypotent myogenic cells in the direction of normal formation of skeletal muscles and provides intensive and long-term nutrition of muscle cells. The application of the peptide IPH AGAA leads to optimization of metabolism in muscle cells, improvement of microcirculation in muscle tissue and restoration of water and mineral balance in muscles. The application of the peptide IPH AGAA has a myoprotective nature, in particular, increasing the activity of actin fibers in the muscle, increasing the contractility and strength of muscle tissue, thereby increasing the elasticity of muscles and providing a stimulating effect on muscles in hypoxia. The data indicate a high onco-protective activity of the peptide IPH AGAA in relation to the cells of the muscles according to the expression of biological molecules in cell culture.
It has also been shown that the peptide IPH AGAA has anti-inflammatory action against muscle tissue damage and thus contributes to the improvement of regeneration of damaged muscles, which is manifested by cytostatic and anti-inflammatory action against muscle cells according to experimental studies. The application of the peptide IPH AGAA reduces the degree of inflammation in muscle tissue damage, improves regeneration and restoration of muscle fibers, prevents the development of contractures and reduce muscle elasticity in the experimental model. These data indicate that the application of the peptide IPH AGAA optimizes metabolism in muscle cells, has an antioxidant effect, prevents damage to muscle cells by free radicals, provides intensive and long-term nutrition of muscle cells, has a stimulating effect on muscles in hypoxia and increases the elasticity of muscles.
Thus, the application of the peptide IPH AGAA significantly improves the subjective assessment of physical activity, energy and endurance in 1.5 times, significantly increases muscle mass in 1.1 times and reduces fat mass in 1.3 times, which indicates the properties of this peptide in terms of normalization of muscle tissue functions and redistribution of muscle mass, that is, changes in body composition. The application of the peptide IPH AGAA significantly improves the performance of dynamometry in 1.2 times compared to the original data. Indicators after 3 months of application of the peptide IPH AGAA reach the upper limit of the norm. Therefore, according to clinical data, the application of the peptide IPH AGAA optimizes metabolism in muscle cells, improves microcirculation in muscle tissue, provides intensive and long-term nutrition of muscle cells, has a stimulating effect on muscles in hypoxia and increases the elasticity of muscles.
The application of peptide IPH AGAA recommended in the form of biologically active food supplement with therapeutic and preventive purposes for the normalization of the system functions of muscles.
With the support of «Ideal Pharma Peptide GMBH», Ferdinandstr. 11 61348 Bad Homburg.
 IPH – PEPTIDES
Read more
IPH – PEPTIDES
Read more