
This article summarizes the potential benefits of melatonin in alleviating COVID-19 based on its presumed pathogenesis. The recent COVID-19 outbreak has turned into a pandemic with tens of thousands of infected patients. Based on the clinical features, pathology, and pathogenesis of acute respiratory distress syndrome (ARDS) caused by highly homogenous coronaviruses or other pathogenic microorganisms, there is evidence suggesting that excessive inflammation, oxidative stress, and an exaggerated immune response likely contribute to COVID-19 pathology. This leads to a cytokine storm and subsequent progression to acute lung injury (ALI) / ARDS and often death. Melatonin, a well-known anti-inflammatory and antioxidant molecule, provides protection against ALI / ARDS induced by viral and other agents. Melatonin is effective in intensive care patients, reducing vascular permeability, anxiety, the use of sedatives, and improving sleep quality, which can also be beneficial for enhancing clinical outcomes in COVID-19 patients. Notably, melatonin has a high safety profile. There is significant data suggesting that melatonin limits virus-related diseases and is likely to be beneficial in COVID-19 patients. Additional experiments and clinical studies are needed to confirm this hypothesis.
Coronaviruses (CoVs) are RNA viruses that infect both humans and animals, affecting the respiratory system, gastrointestinal tract, and central nervous system [1]. Severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) are contagious and deadly, causing thousands of deaths in the past two decades. The recent outbreak was detected in Wuhan, China, and this highly contagious disease has spread throughout China and other countries [2]. While antiviral therapy, corticosteroid treatment, and mechanical respiratory support have been applied, there is no specific treatment for COVID-19 [2].
Melatonin (N-acetyl-5-methoxytryptamine) is a biologically active molecule with a range of health-promoting properties. Melatonin is successfully used to treat sleep disorders, delirium, atherosclerosis, respiratory tract diseases, and viral infections [3]. Previous research has documented the positive effects of melatonin in alleviating acute respiratory stress induced by viruses, bacteria, radiation, and more [4-6]. Here, we will examine the evidence for melatonin’s potential utility as an adjuvant in the treatment of COVID-19 pneumonia, acute lung injury (ALI), and acute respiratory distress syndrome (ARDS).
Patients with COVID-19 (infected with SARS-CoV-2) report symptoms such as fever, dry cough, myalgia, fatigue, diarrhea, and more, with symptoms varying somewhat with the patient’s age. In some cases, the disease’s severe progression leads to ALI / ARDS, respiratory failure, heart failure, sepsis, and sudden cardiac arrest within a few days [2,7]. Pathological examination of lung specimens from moderate COVID-19 patients (who were retrospectively diagnosed with COVID-19 during lung cancer surgery) revealed edema, protein exudate with globules, focal inflammatory cell infiltration, and moderate hyaline membrane formation [8]. Post-mortem examination of a COVID-19 patient with severe ARDS showed bilateral diffuse alveolar damage with edema, pneumocyte desquamation, and hyaline membrane formation [9].
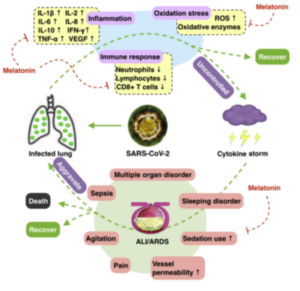
Figure 1. Pathogenesis of COVID-19 and potential adjuvant use of melatonin. We hypothesized that the lungs are infected with SARS-CoV-2, and the suppressed immune response, increased inflammation, and excessive oxidative stress do not subside, leading to the activation of a cytokine storm. ALI / ARDS can follow, accompanied by a range of complications with outcomes varying depending on disease severity. Melatonin may play a role as an adjuvant in regulating the immune system, inflammation, and oxidative stress, as well as providing support to patients with ALI / ARDS and associated complications. ALI: Acute Lung Injury; ARDS: Acute Respiratory Distress Syndrome.
While these pathological findings have been documented in only a small number of cases, they resemble pathological features found in SARS- and MERS-induced pneumonia [10]. SARS-CoVs, MERSCoVs, and SARS-CoV-2 are classified as members of the beta-coronavirus family [11]. Recent published studies show that SARS-CoV-2 shares 79.0% nucleotide identity with SARS-CoV and 51.8% identity with MERSCoV [12], indicating high genetic homology among SARS-CoV-2, MERS-CoV, and SARS-CoV. In animal models infected with SARS-CoV and MERS-CoV, inflammatory and immune reactions are activated, leading to a “cytokine storm” and apoptosis of epithelial and endothelial cells. Subsequently, vascular leakage, abnormal T cells, and macrophages occur. This reaction leads to ALI / ARDS or even death [13].
Based on genetic homology and pathological features of infected individuals, we predicted that COVID-19 patients exhibit certain distinctive characteristics. Notably, in the blood of COVID-19 patients, there was a marked increase in interleukin-1β (IL-1β), interferon gamma (IFN-γ), interferon-inducible protein 10 (IP-10), monocyte chemoattractant protein 1 (MCP-1), as well as IL-4 and IL-10 compared to SARS patients. This suggests some potential differences in the pathogenesis of the coronavirus compared to SARS and MERS [2]. There is also potential immune suppression in COVID-19 patients, characterized by hypoalbuminemia, lymphopenia, neutropenia, and a decreased percentage of CD8+ T cells [2,7]. Recent reports suggest that in some COVID-19 patients, high levels of inflammation are still present even when testing negative for viral nucleic acid. Clinical studies using certolizumab pegol (a TNF blocker) along with other antiviral therapies may have positive effects on COVID-19 patients. Collectively, these findings indicate that inflammation is a key feature of COVID-19 patients. Therefore, we hypothesize that excessive inflammation, immune system depression, and cytokine storm activation significantly contribute to the pathogenesis of COVID-19.
In the early stages of coronavirus infection, dendritic cells and epithelial cells are activated and express pro-inflammatory cytokines and chemokines, including IL-1β, IL-2, IL-6, IL-8, both IFN-α/β, tumor necrosis factor (TNF), C-C motif chemokine ligand 3 (CCL3), CCL5, CCL2, IP-10, and others. They are under the control of the immune system. Therefore, overproduction of these cytokines and chemokines contributes to the development of the disease [14–16]. IL-10, produced by T-helper-2 (Th2) cells, has antiviral properties, leading to a significant reduction in this agent with coronavirus infections [17,18].
Interestingly, some COVID-19 patients sometimes have significantly elevated levels of IL-10 [2]. Whether this is a feature of COVID-19 infection or a result of treatment is unknown. The enhancement of the inflammatory response will lead to cellular apoptosis or necrosis of affected cells, which will further contribute to inflammation, with subsequent increased vascular permeability and abnormal accumulation of inflammatory monocytes, macrophages, and neutrophils in the lung alveoli [19]. This vicious cycle exacerbates the situation as the regulation of the immune response is lost, and the cytokine storm is further activated, leading to severe consequences.
This hypothesized “cytokine storm” pathology associated with coronaviruses is also supported by experimental models of SARS-CoV, one of which showed that the severity of ALI was accompanied by increased expression of genes related to inflammation, rather than an increase in viral titers. In another case, the ablation of the IFN-α/β receptor or depletion of inflammatory monocytes/macrophages resulted in a noticeable increase in the survival of coronavirus hosts without changing the viral load [19,20]. Both situations suggest a potential mechanism for enhancing CoV-induced ALI/ARDS independent of viral load. If a similar pathology also exists in COVID-19, attenuating the cytokine storm by targeting several key steps in the process may lead to improved outcomes.
Melatonin does not possess direct antiviral action but exerts indirect antiviral effects [3] due to its anti-inflammatory, antioxidant, and immune-enhancing properties [21–24]. There are situations where melatonin suppresses features of viral infections. In mice infected with viruses in the central nervous system (e.g., encephalitis), the use of melatonin resulted in lower viremia, reduced paralysis and mortality, and a decrease in viral load [25]. In previous models of respiratory syncytial virus, melatonin caused the suppression of acute oxidative lung damage, pro-inflammatory cytokine release, and recruitment of inflammatory cells. These findings, along with recent summaries by Reiter et al. [3], support the rationale for using melatonin in viral diseases. Furthermore, melatonin’s anti-inflammatory action, antioxidant properties, and immune-stimulating effects contribute to its potential attenuation of COVID-19 infection (Fig. 1).
Melatonin exerts anti-inflammatory action through various pathways. Sirtuin-1 (SIRT1) may mediate melatonin’s anti-inflammatory action by inhibiting high mobility group box 1 (HMGB1) and, thereby, suppressing macrophage polarization toward a pro-inflammatory type [26]. In sepsis-induced ALI, proper regulation of SIRT1 mitigates lung damage and inflammation, in which melatonin use may be beneficial [27]. Nuclear factor kappa-B (NF-κB) is closely associated with pro-inflammatory and pro-oxidative responses, serving as a mediator of inflammation in ALI.
Melatonin’s anti-inflammatory action includes the suppression of NF-κB activation in ARDS [28, 29]. Melatonin, as reported, suppresses NF-κB activation in T cells and lung tissue [30,31]. Stimulation of nuclear factor erythroid 2-related factor 2 (Nrf2) is crucial for protecting the lungs from injury.
In related studies, melatonin induces Nrf2 activation with therapeutic effects in hepatoprotection, cardioprotection, and others [32]. Whether Nrf2 is involved in CoV-induced ALI remains unknown, but the close interaction between SIRT1, NF-κB, and Nrf2 suggests their participation in CoV-induced ALI/ARDS. Thus, data support the potential anti-inflammatory action of melatonin. Inflammation is usually associated with increased cytokine and chemokine production, while melatonin induces a reduction in pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6, and IL-8, as well as an increase in the anti-inflammatory cytokine IL-10 [33,34]. However, there may be concerns about potential pro-inflammatory actions of melatonin when used at very high doses or in immunosuppressed conditions, where it may lead to an increase in the production of pro-inflammatory cytokines like IL-1β, IL-2, IL-6, IL-12, TNF-α, and IFN-γ [35].
Conversely, in ALI infection models, melatonin exhibits anti-inflammatory and protective effects [6].
The antioxidant effect of melatonin interacts with its anti-inflammatory action, enhancing antioxidant enzymes (e.g., superoxide dismutase) and suppressing pro-oxidative enzymes (e.g., nitric oxide synthase). Melatonin can also directly interact with free radicals, acting as a scavenger of free radicals [3,4]. Viral infections and their replication continuously generate oxidative products. In acute respiratory viral infections (ARVIs), the production of oxidized low-density lipoprotein activates the innate immune response through the overproduction of alveolar macrophages’ IL-6 via Toll-like receptor 4 (TLR4) / NF-kB signaling, leading to acute lung injury (ALI) [36]. TLR4 is a receptor of the innate immune system and is also a therapeutic target for melatonin. In conditions such as cerebral ischemia, gastritis, and periodontitis, melatonin has demonstrated its anti-inflammatory effects through TLR4 signaling [37–39]. The antioxidant effect of melatonin has also been confirmed in ALI induced by radiation, sepsis, and ischemia-reperfusion [4,40,41]. In patients with ALI / ARDS, especially as the disease progresses and in patients treated in intensive care units (ICUs) with severe inflammation, hypoxemia, and mechanical ventilation with high concentrations of oxygen, the local and systemic production of oxidants inevitably increases [42,43]. Accordingly, we assume that excessive oxidation also likely participates in COVID-19. Extensive studies by Gitto et al. [44,45], who used melatonin to treat newborns with respiratory distress syndrome, have documented the antioxidant and anti-inflammatory effects of melatonin in the lungs. Therefore, it is quite likely that the use of melatonin would be beneficial in combating inflammation and oxidative stress in individuals infected with the coronavirus.
Although there are no specific reports regarding the use of melatonin in COVID-19 patients, studies involving individuals with other diseases and elevated levels of inflammation have shown promising results regarding the reduction of circulating cytokines. In a randomized controlled trial, an 8-week oral administration of 6 mg/day of melatonin led to a significant decrease in serum levels of IL-6, TNF-α, and hs-C-reactive protein (hs-CRP) in patients with diabetes and periodontitis [56]. In another study involving patients with severe multiple sclerosis, oral administration of 25 mg/day of melatonin for 6 months also contributed to a significant reduction in serum concentrations of TNF-α, IL-6, IL-1β, and lipoperoxides [57]. During the acute phase of inflammation, including surgical stress [58], reperfusion of the brain [59], and coronary artery reperfusion [60], melatonin administration at doses of 10 mg/day, 6 mg/day, and 5 mg/day for less than 5 days resulted in a reduction in proinflammatory cytokine levels. A recent meta-analysis of a total of 22 randomized controlled trials suggested that the supplementary use of melatonin is associated with a significant reduction in TNF-α and IL-6 levels [61].
These clinical data indicate that the use of melatonin as a supplement can effectively reduce circulating cytokine levels and potentially lower levels of proinflammatory cytokines in COVID-19 patients.
The integrity of the vascular endothelial barrier is crucial in immune regulation within the alveoli. Severe inflammation and immune responses lead to apoptosis of epithelial and endothelial cells and an increase in the production of VEGF, exacerbating edema and the extravasation of immune cells from blood vessels. Experimental data suggest that melatonin mediates the suppression of VEGF in vascular endothelial cells [62]. Based on clinical reports in COVID-19, patients with severe forms of acute lung injury (ALI) / acute respiratory distress syndrome (ARDS) may also have an increased risk of sepsis and cardiac arrest [2]. Published reports indicate that melatonin use can mitigate septic shock through the NLRP3 pathway [63]. In particular, melatonin may have a preventive effect against sepsis-induced kidney injury, septic cardiomyopathy, and liver damage [64-66]. It has also been reported that melatonin benefited patients with myocardial infarction, cardiomyopathy, hypertension, and pulmonary hypertension, likely functioning through the TLR4/activating survival factor pathway [67]. Furthermore, melatonin provides neurological protection by reducing brain inflammation, brain edema, and blood-brain barrier permeability in various experimental conditions [68]. In the intensive care unit (ICU), profound sedative effects are associated with increased long-term mortality, and the use of melatonin reduces the use of sedatives and the frequency of pain, agitation, and restlessness [69,70]. Moreover, a recent meta-analysis showed that melatonin improves the quality of sleep in ICU patients [71]. Therefore, the rationale for using melatonin in patients with chronic respiratory insufficiency not only focuses on alleviating respiratory disorders caused by infection but also on overall improvement and the prevention of patient well-being and potential complications.
When considering the use of melatonin for the treatment of COVID-19, the safety of melatonin is of paramount importance. As previously verified, short-term use of melatonin is safe, even at high doses, and reported side effects are limited to occasional dizziness, headache, nausea, and drowsiness; overall, melatonin’s safety profile in humans is very high [72]. In clinical trials, doses of 3 mg, oral intake of 6 mg and 10 mg of melatonin in ICU patients showed satisfactory safety compared to a placebo [70,73,74]. Furthermore, even when melatonin was administered to individuals at a dose of 1 g/day for a month, there were no adverse reports of treatment [75]. Importantly, no side effects were registered in animal studies after melatonin was administered in ALI / ARDS [3,4,28]. While the safety of melatonin has been examined in numerous human studies, its effects when prescribed to COVID-19 patients should be closely monitored, despite melatonin’s very high safety profile.
[1] J. Cui, F. Li, Z.-L. Shi, Origin and evolution of pathogenic coronaviruses, Nat. Rev.
Microbiol. 17 (2019) 181–192, https://doi.org/10.1038/s41579-018-0118-9.
[2] C. Huang, Y. Wang, X. Li, L. Ren, J. Zhao, Y. Hu, L. Zhang, G. Fan, J. Xu, X. Gu,
patients infected with 2019 novel coronavirus in Wuhan, China, Lancet (London,
England) 395 (2020) 497–506, https://doi.org/10.1016/S0140-6736(20)30183-5.
[3] R.J. Reiter, Q. Ma, R. Sharma, Treatment of Ebola and other infectious diseases:
melatonin “goes viral”, Melatonin Res 3 (2020) 43–57, https://doi.org/10.32794/
mr11250047.
[4] X. Wu, H. Ji, Y. Wang, C. Gu, W. Gu, L. Hu, L. Zhu, Melatonin alleviates radiationinduced
lung injury via regulation of miR-30e/NLRP3 axis, Oxidative Med. Cell.
Longev. 2019 (2019) 4087298, https://doi.org/10.1155/2019/4087298.
[5] H.-K. Yip, Y.-C. Chang, C.G. Wallace, L.-T. Chang, T.-H. Tsai, Y.-L. Chen, H.-
treatment improves adipose-derived mesenchymal stem cell therapy for acute lung
ischemia-reperfusion injury, J. Pineal Res. 54 (2013) 207–221, https://doi.org/10.
1111/jpi.12020.
[6] S.-H. Huang, X.-J. Cao, W. Liu, X.-Y. Shi, W. Wei, Inhibitory effect of melatonin on
lung oxidative stress induced by respiratory syncytial virus infection in mice, J.
Pineal Res. 48 (2010) 109–116, https://doi.org/10.1111/j.1600-079X.2009.
00733.x.
[7] N. Chen, M. Zhou, X. Dong, J. Qu, F. Gong, Y. Han, Y. Qiu, J. Wang, Y. Liu, Y. Wei,
cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study,
Lancet (London, England) 395 (2020) 507–513, https://doi.org/10.1016/S0140-
6736(20)30211-7.
[8] S. Tian, W. Hu, L. Niu, H. Liu, H. Xu, S. Xiao, Pulmonary Pathology of Early Phase
SARSCoV-2 Pneumonia, Preprints (Www.Preprints.Org), 2020, https://doi.org/10.
20944/preprints202002.0220.v1 [Epub ahead of print].
[9] Z. Xu, L. Shi, Y. Wang, J. Zhang, L. Huang, C. Zhang, S. Liu, P. Zhao, H. Liu, L. Zhu,
findings of COVID-19 associated with acute respiratory distress syndrome, Lancet
Respir. Med. (2020), https://doi.org/10.1016/S2213-2600(20)30076-X Epub
ahead of print.
[10] J. Liu, X. Zheng, Q. Tong, W. Li, B. Wang, K. Sutter, M. Trilling, M. Lu, U. Dittmer,
emerging human pathogenic coronaviruses SARS-CoV, MERS-CoV, and 2019-nCoV,
[11] J. Chen, Pathogenicity and transmissibility of 2019-nCoV-A quick overview and
comparison with other emerging viruses, Microbes Infect. (2020), https://doi.org/
10.1016/j.micinf.2020.01.004.
[12] L.-L. Ren, Y.-M. Wang, Z.-Q. Wu, Z.-C. Xiang, L. Guo, T. Xu, Y.-Z. Jiang, Y. Xiong, Y.-
J.-W. Wang, Identification of a novel coronavirus causing severe pneumonia in
human: a descriptive study, Chin. Med. J. (2020), https://doi.org/10.1097/CM9.
0000000000000722 Epub ahead of print.
[13] R. Channappanavar, S. Perlman, Pathogenic human coronavirus infections: causes
and consequences of cytokine storm and immunopathology, Semin. Immunopathol.
39 (2017) 529–539, https://doi.org/10.1007/s00281-017-0629-x.
[14] C.Y. Cheung, L.L.M. Poon, I.H.Y. Ng, W. Luk, S.-F. Sia, M.H.S. Wu, K.-H. Chan, K.-
syndrome coronavirus-infected macrophages in vitro: possible relevance
to pathogenesis, J. Virol. 79 (2005) 7819–7826, https://doi.org/10.1128/JVI.79.
12.7819-7826.2005.
[15] H.K.W. Law, C.Y. Cheung, H.Y. Ng, S.F. Sia, Y.O. Chan, W. Luk, J.M. Nicholls,
J.S.M. Peiris, Y.L. Lau, Chemokine up-regulation in SARS-coronavirus-infected,
monocyte-derived human dendritic cells, Blood 106 (2005) 2366–2374, https://
doi.org/10.1182/blood-2004-10-4166.
[16] H. Chu, J. Zhou, B.H.-Y. Wong, C.C. Li, J.F.-W. Chan, Z.-S. Cheng, D. Yang, D. Wang,
A.C.-Y. Lee, C.C. Li, M.-L. Yeung, J.-P. Cai, I.H.-Y. Chan, W.-K. Ho, K.K.-W. To, B.-
efficiently infects human primary T lymphocytes and activates the extrinsic and
intrinsic apoptosis pathways, J. Infect. Dis. 213 (2016) 904–914, https://doi.org/
10.1093/infdis/jiv380.
[17] A.R. Fehr, R. Channappanavar, G. Jankevicius, C. Fett, J. Zhao, J. Athmer,
D.K. Meyerholz, I. Ahel, S. Perlman, The conserved coronavirus macrodomain
promotes virulence and suppresses the innate immune response during severe acute
respiratory syndrome coronavirus infection, MBio 7 (2016), https://doi.org/10.
1128/mBio.01721-16.
[18] J.-Y. Chien, P.-R. Hsueh, W.-C. Cheng, C.-J. Yu, P.-C. Yang, Temporal changes in
cytokine/chemokine profiles and pulmonary involvement in severe acute respiratory
syndrome, Respirology (Carlton, Vic.) 11 (2006) 715–722, https://doi.
org/10.1111/j.1440-1843.2006.00942.x.
[19] R. Channappanavar, A.R. Fehr, R. Vijay, M. Mack, J. Zhao, D.K. Meyerholz,
responses cause lethal pneumonia in SARS-CoV-infected mice, Cell Host
Microbe 19 (2016) 181–193, https://doi.org/10.1016/j.chom.2016.01.007.
[20] S.L. Smits, A. de Lang, J.M.A. van den Brand, L.M. Leijten, W.F. van IJcken,
M.J.C. Eijkemans, G. van Amerongen, T. Kuiken, A.C. Andeweg,
A.D.M.E. Osterhaus, B.L. Haagmans, Exacerbated innate host response to SARS-CoV
in aged non-human primates, PLoS Pathog. 6 (2010) e1000756–e1000756, ,
https://doi.org/10.1371/journal.ppat.1000756.
[21] A. Junaid, H. Tang, A. van Reeuwijk, Y. Abouleila, P. Wuelfroth, V. van Duinen,
shock syndrome-on-a-chip, IScience 23 (2020) 100765, , https://doi.org/10.1016/j.
isci.2019.100765.
[22] J.A. Boga, A. Coto-Montes, S.A. Rosales-Corral, D.-X. Tan, R.J. Reiter, Beneficial
actions of melatonin in the management of viral infections: a new use for this
“molecular handyman”? Rev. Med. Virol. 22 (2012) 323–338, https://doi.org/10.
1002/rmv.1714.
[23] G. Anderson, M. Maes, R.P. Markus, M. Rodriguez, Ebola virus: melatonin as a
readily available treatment option, J. Med. Virol. 87 (2015) 537–543, https://doi.
org/10.1002/jmv.24130.
[24] R.J. Reiter, Q. Ma, R. Sharma, Melatonin in mitochondria: mitigating clear and
present dangers, Physiology (Bethesda) 35 (2020) 86–95, https://doi.org/10.1152/
physiol.00034.2019.
[25] D. Ben-Nathan, G.J. Maestroni, S. Lustig, A. Conti, Protective effects of melatonin in
mice infected with encephalitis viruses, Arch. Virol. 140 (1995) 223–230, https://
doi.org/10.1007/bf01309858.
[26] R. Hardeland, Melatonin and inflammation-story of a double-edged blade, J. Pineal
Res. 65 (2018) e12525, https://doi.org/10.1111/jpi.12525.
[27] Q.-L. Wang, L. Yang, Y. Peng, M. Gao, M.-S. Yang, W. Xing, X.-Z. Xiao, Ginsenoside
Rg1 regulates SIRT1 to ameliorate sepsis-induced lung inflammation and injury via
inhibiting endoplasmic reticulum stress and inflammation, Mediat. Inflamm. 2019
(2019) 6453296, https://doi.org/10.1155/2019/6453296.
[28] C.-K. Sun, F.-Y. Lee, Y.-H. Kao, H.-J. Chiang, P.-H. Sung, T.-H. Tsai, Y.-C. Lin, S. Leu,
Y.-C. Wu, H.-I. Lu, Y.-L. Chen, S.-Y. Chung, H.-L. Su, H.-K. Yip, Systemic combined
melatonin-mitochondria treatment improves acute respiratory distress syndrome in
the rat, J. Pineal Res. 58 (2015) 137–150, https://doi.org/10.1111/jpi.12199.
[29] Y. Ling, Z.-Z. Li, J.-F. Zhang, X.-W. Zheng, Z.-Q. Lei, R.-Y. Chen, J.-H. Feng,
MicroRNA-494 inhibition alleviates acute lung injury through Nrf2 signaling
pathway via NQO1 in sepsis-associated acute respiratory distress syndrome, Life
Sci. 210 (2018) 1–8, https://doi.org/10.1016/j.lfs.2018.08.037.
[30] A.M. da C. Pedrosa, R. Weinlich, G.P. Mognol, B.K. Robbs, J.P. de B. Viola,
induced cell death by blocking NFAT-mediated CD95 ligand upregulation, J.
Immunol (Baltimore, Md.: 1950) 184 (2010) 3487–3494, https://doi.org/10.4049/
jimmunol.0902961.
[31] Y. Shang, S.-P. Xu, Y. Wu, Y.-X. Jiang, Z.-Y. Wu, S.-Y. Yuan, S.-L. Yao, Melatonin
reduces acute lung injury in endotoxemic rats, Chin. Med. J. 122 (2009)
1388–1393.
[32] Z. Ahmadi, M. Ashrafizadeh, Melatonin as a potential modulator of Nrf2, Fund.
Clin. Pharmacol. 34 (2020) 11–19, https://doi.org/10.1111/fcp.12498.
[33] S. Habtemariam, M. Daglia, A. Sureda, Z. Selamoglu, M.F. Gulhan, S.M. Nabavi,
Melatonin and respiratory diseases: a review, Curr. Top. Med. Chem. 17 (2017)
467–488, https://doi.org/10.2174/1568026616666160824120338.
[34] R. Hardeland, Aging, melatonin, and the pro- and anti-inflammatory networks, Int.
[35] L. Carrascal, P. Nunez-Abades, A. Ayala, M. Cano, Role of melatonin in the inflammatory
process and its therapeutic potential, Curr. Pharm. Design. 24 (2018)
1563–1588, https://doi.org/10.2174/1381612824666180426112832.
[36] Y. Imai, K. Kuba, G.G. Neely, R. Yaghubian-Malhami, T. Perkmann, G. van Loo,
oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung
injury, Cell 133 (2008) 235–249, https://doi.org/10.1016/j.cell.2008.02.043.
[37] Y. Zhao, H. Wang, W. Chen, L. Chen, D. Liu, X. Wang, X. Wang, Melatonin attenuates
white matter damage after focal brain ischemia in rats by regulating the
TLR4/NF-kappaB pathway, Brain Res. Bull. 150 (2019) 168–178, https://doi.org/
10.1016/j.brainresbull.2019.05.019.
[38] J. Luo, J. Song, H. Zhang, F. Zhang, H. Liu, L. Li, Z. Zhang, L. Chen, M. Zhang,
production via the TLR2 and TLR4 pathways in H. pylori infected mice, Int.
Immunopharmacol. 64 (2018) 116–122, https://doi.org/10.1016/j.intimp.2018.
08.034.
[39] T.-Y. Renn, Y.-K. Huang, S.-W. Feng, H.-W. Wang, W.-F. Lee, C.-T. Lin, T. Burnouf,
L.-Y. Chen, P.-F. Kao, H.-M. Chang, Prophylactic supplement with melatonin successfully
suppresses the pathogenesis of periodontitis through normalizing RANKL/
OPG ratio and depressing the TLR4/MyD88 signaling pathway, J. Pineal Res. 64
(2018), https://doi.org/10.1111/jpi.12464.
[40] H.-H. Chen, C.-L. Chang, K.-C. Lin, P.-H. Sung, H.-T. Chai, Y.-Y. Zhen, Y.-C. Chen,
Y.-C. Wu, S. Leu, T.-H. Tsai, C.-H. Chen, H.-W. Chang, H.-K. Yip, Melatonin augments
apoptotic adipose-derived mesenchymal stem cell treatment against sepsisinduced
acute lung injury, Am. J. Transl. Res. 6 (2014) 439–458.
[41] M.-L. Wang, C.-H. Wei, W.-D. Wang, J.-S. Wang, J. Zhang, J.-J. Wang, Melatonin
attenuates lung ischaemia-reperfusion injury via inhibition of oxidative stress and
inflammation, Interact. Cardiov. Th. 26 (2018) 761–767, https://doi.org/10.1093/
icvts/ivx440.
[42] D.Y. Tamura, E.E. Moore, D.A. Partrick, J.L. Johnson, P.J. Offner, C.C. Silliman,
Acute hypoxemia in humans enhances the neutrophil inflammatory response, Shock
(Augusta, Ga.) 17 (2002) 269–273, https://doi.org/10.1097/00024382-
200204000-00005.
[43] J.V. Sarma, P.A. Ward, Oxidants and redox signaling in acute lung injury, Compr.
Physiol. 1 (2011) 1365–1381, https://doi.org/10.1002/cphy.c100068.
[44] E. Gitto, R.J. Reiter, G. Sabatino, G. Buonocore, C. Romeo, P. Gitto, C. Buggé,
and modality of ventilation in preterm newborns: improvement with melatonin
treatment, J. Pineal Res. 39 (2005) 287–293, https://doi.org/10.1111/j.1600-
079X.2005.00251.x.
[45] E. Gitto, R.J. Reiter, S.P. Cordaro, R.M. La, P. Chiurazzi, G. Trimarchi, P. Gitto,
M.P. Calabrò, I. Barberi, Oxidative and inflammatory parameters in respiratory
distress syndrome of preterm newborns: beneficial effects of melatonin, Am. J.
Perinatol. 21 (2004) 209–216, https://doi.org/10.1055/s-2004-828610.
[46] M.C. Rogers, J.V. Williams, Quis Custodiet Ipsos Custodes? Regulation of cellmediated
immune responses following viral lung infections, Annu. Rev. Virol. 5
(2018) 363–383, https://doi.org/10.1146/annurev-virology-092917-043515.
[47] C.-Y. Yang, C.-S. Chen, G.-T. Yiang, Y.-L. Cheng, S.-B. Yong, M.-Y. Wu, C.-J. Li, New
insights into the immune molecular regulation of the pathogenesis of acute respiratory
distress syndrome, Int. J. Mol. Sci. 19 (2018), https://doi.org/10.3390/
ijms19020588.
[48] Y. Liu, Y. Yang, C. Zhang, F. Huang, F. Wang, J. Yuan, Z. Wang, J. Li, J. Li, C. Feng,
linked to viral loads and lung injury, Sci. China Life Sci. (2020), https://doi.org/10.
1007/s11427-020-1643-8.
[49] S.C. Miller, S.R. Pandi-Perumal, A.I. Esquifino, D.P. Cardinali, G.J.M. Maestroni,
The role of melatonin in immuno-enhancement: potential application in cancer, Int.
00474.x.
[50] C. Kaur, E.A. Ling, Effects of melatonin on macrophages/microglia in postnatal rat
brain, J. Pineal Res. 26 (1999) 158–168, https://doi.org/10.1111/j.1600-079x.
1999.tb00578.x.
[51] M.D. Tate, J.D.H. Ong, J.K. Dowling, J.L. McAuley, A.B. Robertson, E. Latz,
G.R. Drummond, M.A. Cooper, P.J. Hertzog, A. Mansell, Reassessing the role of the
NLRP3 inflammasome during pathogenic influenza a virus infection via temporal
inhibition, Sci. Rep. 6 (2016) 27912, https://doi.org/10.1038/srep27912.
[52] C. Shen, Z. Zhang, T. Xie, J. Ji, J. Xu, L. Lin, J. Yan, A. Kang, Q. Dai, Y. Dong,
human respiratory syncytial virus through inhibiting NLRP3 inflammasome activation
via NF-kappaB pathway in mice, Front. Pharmacol. 10 (2019) 1600, https://
doi.org/10.3389/fphar.2019.01600.
R.
[53] S.H.J. Mei, S.D. McCarter, Y. Deng, C.H. Parker, W.C. Liles, D.J. Stewart, Prevention
of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing
angiopoietin 1, PLoS Med. 4 (2007) e269, https://doi.org/10.1371/journal.pmed.
0040269.
[54] H.-M. Wu, Q.-M. Xie, C.-C. Zhao, J. Xu, X.-Y. Fan, G.-H. Fei, Melatonin biosynthesis
restored by CpG oligodeoxynucleotides attenuates allergic airway inflammation via
regulating NLRP3 inflammasome, Life Sci. 239 (2019) 117067, https://doi.org/10.
1016/j.lfs.2019.117067.
[55] Y. Zhang, X.X. Li, J.J. Grailer, N. Wang, M. Wang, J. Yao, R. Zhong, G.F. Gao,
P.A. Ward, D.-X. Tan, X.X. Li, Melatonin alleviates acute lung injury through inhibiting
the NLRP3 inflammasome, J. Pineal Res. 60 (2016) 405–414, https://doi.
org/10.1111/jpi.12322.
[56] H. Bazyar, H. Gholinezhad, L. Moradi, P. Salehi, F. Abadi, M. Ravanbakhsh, A. Zare
Javid, The effects of melatonin supplementation in adjunct with non-surgical periodontal
therapy on periodontal status, serum melatonin and inflammatory markers
in type 2 diabetes mellitus patients with chronic periodontitis: a double-blind,
placebo-controlled trial, Inflammopharmacology 27 (2019) 67–76, https://doi.org/
10.1007/s10787-018-0539-0.
[57] A.L. Sanchez-Lopez, G.G. Ortiz, F.P. Pacheco-Moises, M.A. Mireles-Ramirez,
O.K. Bitzer-Quintero, D.L.C. Delgado-Lara, L.J. Ramirez-Jirano, I.E. Velazquez-
Brizuela, Efficacy of melatonin on serum pro-inflammatory cytokines and oxidative
stress markers in relapsing remitting multiple sclerosis, Arch. Med. Res. 49 (2018)
391–398, https://doi.org/10.1016/j.arcmed.2018.12.004.
[58] B. Kucukakin, J. Lykkesfeldt, H.J. Nielsen, R.J. Reiter, J. Rosenberg, I. Gogenur,
Utility of melatonin to treat surgical stress after major vascular surgery–a safety
study, J. Pineal Res. 44 (2008) 426–431, https://doi.org/10.1111/j.1600-079X.
2007.00545.x.
[59] Z. Zhao, C. Lu, T. Li, W. Wang, W. Ye, R. Zeng, L. Ni, Z. Lai, X. Wang, C. Liu, The
protective effect of melatonin on brain ischemia and reperfusion in rats and humans:
in vivo assessment and a randomized controlled trial, J. Pineal Res. 65 (2018)
e12521, https://doi.org/10.1111/jpi.12521.
[60] E. Shafiei, M. Bahtoei, P. Raj, A. Ostovar, D. Iranpour, S. Akbarzadeh, H. Shahryari,
and melatonin on early reperfusion injury in patients undergoing coronary
artery bypass grafting: a randomized, open-labeled, placebo-controlled trial,
Medicine. 97 (2018) e11383, https://doi.org/10.1097/MD.0000000000011383.
[61] M. Zarezadeh, M. Khorshidi, M. Emami, P. Janmohammadi, H. Kord-Varkaneh,
S.M. Mousavi, S.H. Mohammed, A. Saedisomeolia, S. Alizadeh, Melatonin supplementation
and pro-inflammatory mediators: a systematic review and meta-analysis
of clinical trials, Eur. J. Nutr. (2019), https://doi.org/10.1007/s00394-019-02123-
0 [Epub ahead of print].
[62] J. Cheng, H.-L. Yang, C.-J. Gu, Y.-K. Liu, J. Shao, R. Zhu, Y.-Y. He, X.-Y. Zhu, M.-
by suppressing HIF-1alpha/ROS/VEGF, Int. J. Mol. Med. 43 (2019) 945–955,
https://doi.org/10.3892/ijmm.2018.4021.
[63] H. Volt, J.A. Garcia, C. Doerrier, M.E. Diaz-Casado, A. Guerra-Librero, L.C. Lopez,
expression: aging and sepsis trigger NLRP3 inflammasome activation, a target of
melatonin, J. Pineal Res. 60 (2016) 193–205, https://doi.org/10.1111/jpi.12303.
[64] W. Dai, H. Huang, L. Si, S. Hu, L. Zhou, L. Xu, Y. Deng, Melatonin prevents sepsisinduced
renal injury via the PINK1/Parkin1 signaling pathway, Int. J. Mol. Med. 44
(2019) 1197–1204, https://doi.org/10.3892/ijmm.2019.4306.
[65] J. Zhang, L. Wang, W. Xie, S. Hu, H. Zhou, P. Zhu, H. Zhu, Melatonin attenuates ER
stress and mitochondrial damage in septic cardiomyopathy: a new mechanism involving
BAP31 upregulation and MAPK-ERK pathway, J. Cell. Physiol. 235 (2020)
2847–2856, https://doi.org/10.1002/jcp.29190.
[66] J. Chen, H. Xia, L. Zhang, H. Zhang, D. Wang, X. Tao, Protective effects of melatonin
on sepsis-induced liver injury and dysregulation of gluconeogenesis in rats through
activating SIRT1/STAT3 pathway, Biomed. Pharmacother. 117 (2019) 109150,
https://doi.org/10.1016/j.biopha.2019.109150.
[67] F. Nduhirabandi, K. Lamont, Z. Albertyn, L.H. Opie, S. Lecour, Role of toll-like
receptor 4 in melatonin-induced cardioprotection, J. Pineal Res. 60 (2016) 39–47,
https://doi.org/10.1111/jpi.12286.
[68] S. Tordjman, S. Chokron, R. Delorme, A. Charrier, E. Bellissant, N. Jaafari,
Neuropharmacol. 15 (2017) 434–443, https://doi.org/10.2174/
1570159X14666161228122115.
[69] K. Lewandowska, M.A. Malkiewicz, M. Sieminski, W.J. Cubala, P.J. Winklewski,
W.A. Medrzycka-Dabrowska, The role of melatonin and melatonin receptor agonist
in the prevention of sleep disturbances and delirium in intensive care unit — a
clinical review, Sleep Med. 69 (2020) 127–134, https://doi.org/10.1016/j.sleep.
2020.01.019.
[70] G. Mistraletti, M. Umbrello, G. Sabbatini, S. Miori, M. Taverna, B. Cerri,
E.S. Mantovani, P. Formenti, P. Spanu, A. D’Agostino, S. Salini, A. Morabito,
ICU patients: a randomized controlled trial, Minerva Anestesiol. 81 (2015)
1298–1310.
[71] S.R. Lewis, M.W. Pritchard, O.J. Schofield-Robinson, P. Alderson, A.F. Smith,
Melatonin for the promotion of sleep in adults in the intensive care unit, The
Cochrane Database of Syst. Rev. 5 (2018) CD012455, https://doi.org/10.1002/
14651858.CD012455.pub2.
[72] L.P.H. Andersen, I. Gogenur, J. Rosenberg, R.J. Reiter, The safety of melatonin in
humans, Clin. Drug Investig. 36 (2016) 169–175, https://doi.org/10.1007/s40261-
015-0368-5.
[73] R.S. Bourne, G.H. Mills, C. Minelli, Melatonin therapy to improve nocturnal sleep in
critically ill patients: encouraging results from a small randomised controlled trial,
Crit. Care (London, England). 12 (2008) R52, https://doi.org/10.1186/cc6871.
[74] G. Mistraletti, G. Sabbatini, M. Taverna, M.A. Figini, M. Umbrello, P. Magni,
patients, J. Pineal Res. 48 (2010) 142–147, https://doi.org/10.1111/j.1600-079X.
2009.00737.x.
[75] J.J. Nordlund, A.B. Lerner, The effects of oral melatonin on skin color and on the
release of pituitary hormones, J. Clin. Endocrinol. Metab. 45 (1977) 768–774,
https://doi.org/10.1210/jcem-45-4-768.