
Modern medicine places great emphasis on the quality of life. Men experiencing hormonal changes have a particularly reduced quality of life [1].
The increased life expectancy of men is accompanied by an increase in age-related clinical signs and symptoms such as muscle weakness, osteoporosis, the development of benign prostatic hyperplasia, body composition changes, fatigue, reduced sexual interest and activity, as well as an increased prevalence of erectile dysfunction, all of which limit the quality of life. This condition is known as age-related androgen deficiency. Many of these symptoms are similar to those of clinically apparent hormone deficiencies, such as Kallmann syndrome, Prader-Willi syndrome, or deficiencies caused by pituitary tumor treatments. The beneficial effects of hormone replacement therapy in men with age-related androgen deficiency and in postmenopausal women have raised hopes that hormone replacement therapy could prevent or even reverse some of the symptoms of male aging. However, this approach is hindered by the lack of individual age-specific hormone reference values and reliable clinical indicators. Data available to date do not support the need for widespread hormone replacement in elderly men. More extensive long-term prospective studies are needed to determine clinically useful parameters, and then to demonstrate that hormone replacement therapy can lead to functional improvements, thus providing individual benefits from treating aging men. It is also important to find non-hormonal drugs that could normalize the hormonal background [2].
Advances in medical care, such as vaccination and the advent of antibiotics, have dramatically increased the average lifespan in most industrialized countries. Unfortunately, this progress is accompanied by age-related pathological and premorbid changes such as muscle weakness, general frailty, osteoporosis, or benign prostatic hyperplasia (prostate adenoma), which limit the free and independent life of elderly men. Consequently, the main task of modern society should not be just to prolong life, but to extend independent and healthy life, thereby improving the quality of life and, as a byproduct, reducing the costs of caring for the elderly.
During aging and intercurrent pathological processes, physical performance gradually declines in both genders. Among other changes, aging in men is characterized by a decrease in sexual interest and activity, as well as an increased prevalence of erectile dysfunction, as shown by the Massachusetts Male Aging Study. Additionally, elderly men may suffer from several physical and emotional symptoms, some of which are accompanied by hormonal changes similar to those observed in women during the perimenopausal period [1,3].
The loss of female cyclicity and reproductive function, i.e., menopause, is a universal phenomenon at the age of about 50. By the age of 40, the frequency of ovulations decreases, and the reproductive function of the ovaries ceases over the next 15 years. Since men generally do not experience a rapid decline in gonadal function or irreversible loss of reproductive capacity in old age, the term “andropause” is inappropriate. Nevertheless, a gradual decline in serum hormone levels can be observed. The decrease in testosterone levels in serum blood during aging is characterized by high interindividual variability, thus not all aging men reach a clinically significant degree of hypogonadism. In addition to testosterone, three other hormones have attracted attention as potential candidates for hormone replacement therapy in men who are approaching or already in old age: growth hormone, dehydroepiandrosterone (DHEA), and melatonin, their prevalence in the popular press is inversely proportional to their scientifically proven benefits for elderly men [4].
In 1990, Rudman suggested that growth hormone could reverse some aging-related symptoms. Unfortunately, his longer study showed that side effects such as carpal tunnel syndrome could limit the long-term use of growth hormone. To circumvent this complication, oral growth hormone secretion stimulants were developed, releasing physiological amounts of growth hormone from the pituitary gland. The clinical usefulness of drugs that increase growth hormone secretion has not yet been proven. Safety concerns regarding the administration of growth hormone are particularly significant since it has been shown that middle-aged people with higher levels of growth hormone are more likely to die earlier than those with lower levels. Moreover, a study using mice with genetic defects in growth hormone secretion showed that reduced growth hormone secretion could prolong survival. The results of controlled clinical trials reported so far do not support the concept that growth hormone replacement therapy would significantly improve the daily function of elderly men [2].
There are only a few scientific reports on the use of DHEA for replacement therapy. Due to the small number of subjects and the short duration of hormone replacement therapy, insufficient data have been collected regarding the long-term safety or beneficial effects of DHEA. Despite its impressive age-dependent decrease, it is still impossible to determine whether it affects the clinical symptoms of elderly men.
It is a proven fact that testosterone levels in serum blood decrease with age in men. Many clinical signs accompanying the aging process, such as reduced muscle mass, strength, and energy, reduced masculinity, libido, and sexual activity, increased frequency of impotence, reduced cognitive functions, and worsening overall well-being, are also observed in young men with androgen deficiency. This has led to speculation that lower androgen levels in aging men may be responsible for some of these changes and that androgen replacement therapy could prevent, slow down, or even reverse some of them [3,4].
This article will provide a brief overview of the research findings on the effects of using the peptide IPH LGA, which may contribute to the normalization of male hormonal background and enhance the quality of life. The increase in local and systemic biomarker levels, released upon peptide application and possessing protective properties, indicates the importance of their use in terms of preserving and restoring organ functions at any life stage and in any diseases [5,6].
Objective: To explore the potentials of the peptide complex IPH LGA.
The conducted study evaluated the molecular-cellular impact, the effect on biological age and aging markers, and the clinical efficacy of the peptide IPH LGA as a protector of the male hormonal background. We examined patients with age-related androgen deficiency (n=45 patients, 49.7±1.2 years) and a control group (n=42 individuals, 49.9±1.2 years).
All patients were examined before surgery according to the standards of providing urological care to the population (Order of the Ministry of Health of Russia from 12.11.2012 No. 907n). Biochemical analysis was conducted on the automatic biochemical analyzer XL100 (ErbaLachema). The Beck Depression Inventory was chosen to assess depressive disorders. The SF-36 questionnaire was used to evaluate the quality of life.
We used German peptides IPH LGA, which have all the necessary approvals and permissions for global markets, such as: WADA certificate (anti-doping), MAFFA certificate (safety), ORGANIC certificate, HALAL certificate, patent protection: a patent in the United States – Patent Application Publication (United States, No.:US2021/052534A1, date: Feb.25,2021), patent in the European Union No. 016704471, patent in the Russian Federation No. 645608, patent in the People’s Republic of China No. 30507522.
Effectiveness of the peptide IPH LGA application after 3 months.
In processing the study data, the calculation of mean intensive and extensive values was carried out with the calculation of the standard error; the significance of differences between two groups was assessed using the Student’s t-test (the difference in indicators was considered significant at t>2, p<0.05).
Results and Discussion: Protective Properties of the Peptide IPH LGA at the Gene-Molecular Level
With aging, a gradual decrease in the level of male hormones in the blood serum, such as testosterone, dehydroepiandrosterone, and growth hormone, can be observed. However, this decrease can be seen at an early age due to disturbances during embryogenesis, affecting health.
To evaluate the cytostatic and oncoprotective properties of the peptide IPH LGA in relation to the male hormonal background, we chose embryonic stem cells.
Optimal functioning and tuning of STR TAAAA(n), STR (CAG)n gene polymorphisms lead to the proper functioning of the hormonal background. The cytoprotective abilities of the peptide IPH LGA were studied through the expression of STR TAAAA(n), STR (CAG)n gene polymorphisms (Figure 1).
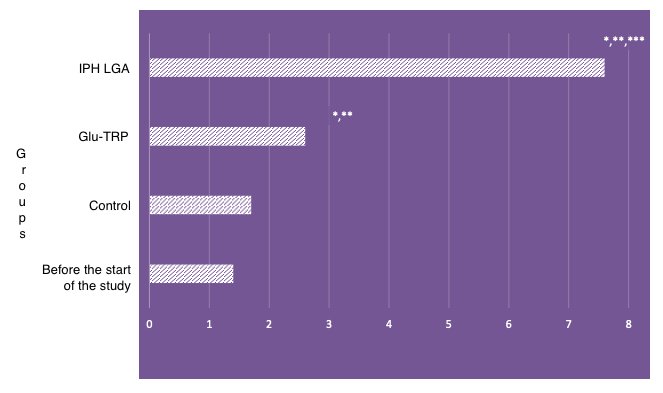
*p<0.05 compared to baseline data;
**p<0.05 compared to control;
***p<0.05 between expression level indicators when using Glu-Trp and IPH LGA.
Figure 1. Expression of STR TAAAA(n), STR (CAG)n gene polymorphisms
The effect of the peptide IPH LGA on the expression of STR TAAAA(n), STR (CAG)n genes was significant, enhancing the expression of the normal genetic apparatus by 58.3%. Such results demonstrate oncoprotective and cytostatic properties.
The impact of the peptide IPH LGA on the expression of embryonic stem cells’ embryonic antigen-4 SSEA-4 (813-70) PE, an increase in which may indicate the development of oncological processes, and the p53 protein in cell cultures, which is a suppressor of oncological processes (Figure 2).
The effect of the peptide IPH LGA on reducing the expression of SSEA-4 (813-70) PE by 49.8% confirms the oncoprotective property of the peptide, which is important for protecting against the progression of prostate cancer and hormone-dependent tumors. Thus, the application of the peptide IPH LGA has a pronounced oncoprotective character, especially in malignant neoplasms of the genitourinary system.
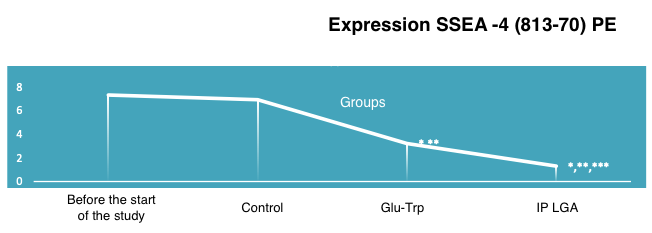
*p<0.05 compared to baseline data;
**p<0.05 compared to control;
***p<0.05 between expression level indicators when using Glu-Trp and IPH LGA.
Figure 2. The effect of the peptide IPH LGA on the expression of SSEA-4 (813-70) PE in human cell culture
The increase in the expression of the p53 protein when using the peptide IPH LGA prevents the development of genetic apparatus mutations, allowing embryogenesis to avoid hormonal background imbalances. The increase in the expression of the p53 protein when using the peptide IPH LGA by 69.8% indicates high cytoprotection and oncoprotection (Figure 3).
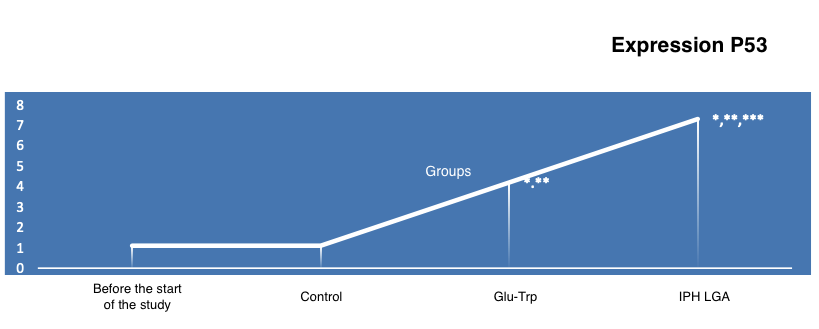
*p<0.05 compared to baseline data;
**p<0.05 compared to control;
***p<0.05 between expression level indicators when using Glu-Trp and IPH LGA.
Figure 3. The effect of the peptide IPH LGA on the expression of the p53 protein in human cell culture
The obtained data indicate the high oncoprotective activity of the peptide IPH LGA concerning the cells of the male reproductive system according to the expression of biological molecules in cell culture.
These experimental data allowed us to determine changes in laboratory data, significantly altered in the presence of age-related androgen deficiency.
Important results were obtained regarding the normalization of the male hormonal background, showing that three months after the application of the peptide IPH LGA, the growth hormone levels were restored to the maximum possible reference values, up to 4.9 ng/ml, and the testosterone level to 6.9±0.8 ng/ml, which is 87.4% higher than before and in the control group (Table 1).
Table 1: Dynamics of sex hormone levels in the blood serum after the application of the peptide biological process regulator IPH LGA in patients with age-related androgen deficiency
| Indicator | Control Group Before | Control Group After 3 Months | Main Group Before | Main Group After 3 Months |
|---|---|---|---|---|
| Growth Hormone, ng/ml | 1.1±0.14 | 1.2±0.14 | 1.1±0.14 | 4.9±0.45 |
| DHEA, μg/dl | 123.71±0.56 | 165.34±0.67 | 131.25±0.57 | 3890.78±1.45 |
| LH, ng/ml | 3.71±0.14 | 3.52±0.12 | 3.09±0.09 | 3.10±0.09 |
| FSH, ng/ml | 2.96±0.18 | 2.69±0.15 | 2.22±0.14 | 2.20±0.14 |
| Testosterone, ng/ml | 2.3±0.4 | 2.6±0.6 | 2.9±0.8 | 6.9±0.8* |
These data allow the use of the peptide IPH LGA as a prophylactic and therapeutic drug for normalizing the male hormonal background in age-related androgen deficiency.
We further studied the quality of the spermogram after the application of the peptide IPH LGA. The use of the peptide IPH increased both the concentration of spermatozoa by 48.6% and the number of active cells by 47.8% (Figure 4).
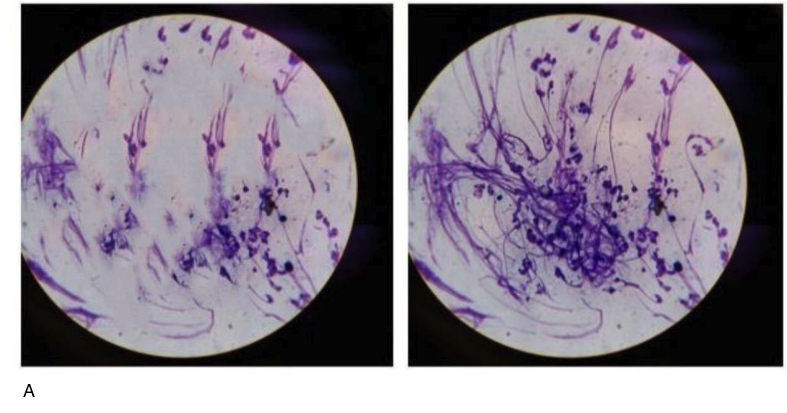
A – before the application of the peptide IPH LGA
B – after the application of the peptide IPH LGA
Figure 4. Spermogram microscopy (before and after the application of the peptide IPH LGA)
Thus, the application of the peptide IPH LGA has a protective effect on testicular cells and increases fertility by enhancing the activity and quantity of spermatozoa.
Such normalization of the hormonal background after the application of the peptide IPH LGA led to an improvement in mood, emotional background stability, an increase in physical health, and work capacity in men by 56.7%, 67.4%, 59.2%, and 45.7%, respectively, as shown in the study of quality of life using the SF-36 questionnaire (Figure 5).
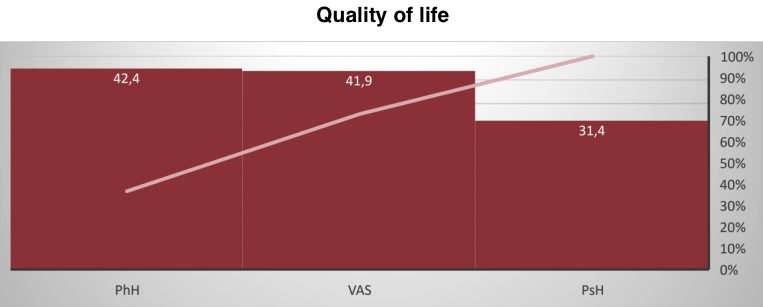
Figure 5. Assessment of quality of life components (scores, M±m)
The obtained data indicate that the peptide IPH LGA acts as an agent leading to the normalization of the hormonal background in age-related changes in men, thereby improving the quality of life.
1. Ilnickii A.N., Prashchayeu K.I. Neujazvimye. Kniga o zdorov’e [Invulnerable. The book
about health]. M.: Diskurs [Discourse]; 2021. 336 p.
2. Dipla K, et al. Relative energy deficiency in sports (RED-S): elucidation of endocrine
changes affecting the health of males and females. Hormones (Athens). 2021; 3: 325-334.
3. Casto KV, et al. Testosterone, cortisol, and human competition. Horm Behav. 2016; 6:
271-305.
4. D’hoore L, et al. Gender-affirming hormone therapy: An updated literature review with an
eye on the future. J Intern Med. 2022; 89: 498-504.
5. Havinson V.H., Lin’kova N.S., Kvetnoj I.M. et al. Molekuljarno-kletochnye mehanizmy
peptidnoj reguljacii sinteza melatonina v kul’ture pinelocitov [Molecular and cellular mechanisms
of peptide regulation of melatonin synthesis in pinealocyte culture]. Byulleten’ eksperimental’noj
biologii i mediciny [Bulletin of Experimental Biology and Medicine]. 2012; 153 (2):223–226.
6. Trofimova S.V., Lin’kova S.N., Klimenko A.A. et al. Pineamin povyshaet sintez
melatonina v jepifize u lic pozhilogo vozrasta [Pineamin increased pineal melatonin synthesis in
elderly people]. Uspehi gerontolii [Advances in Gerontology]. 2017; 30(3):422–426.